What is Arsenic?
Arsenic has been known as the “king of poisons” since the Roman Empire and Victorian era. yes, it has gained a reputation as a lethal substance, but its usefulness in various applications makes it an important chemical element. You’ll find out more about this intriguing element here.
Where is Arsenic obtained?
Arsenic is mainly found in minerals with arsenopyrite containing the most arsenic. Other minerals that contain arsenic include enargite, orpiment, and realgar. You can also get arsenic from the byproduct of copper and lead refining.

The earth’s crust contains arsenic too. It is rare to find arsenic in free form. In the industrial setting, arsenic is obtained from silver, copper, and gold byproduct.
History of Arsenic
In the ancient times, such as in Chinese, Greek, and Egyptian civilizations, arsenic compounds were mined, but its use is not that well-established. Although, it has been used to purposely kill someone, hence, explains why it is called the king of poisons. However, it is believed that arsenic was discovered in 1250 by Albertus Magnus, a German alchemist.

Classification, Properties, and Characteristics of Arsenic
Arsenic is a steel gray/silvery gray solid known for its brittleness and low electrical and thermal conductivity. Some forms of arsenic are metal-like, although there are forms that up to this date are not properly characterized. Arsenic sublimes at 613 degrees Celsius.
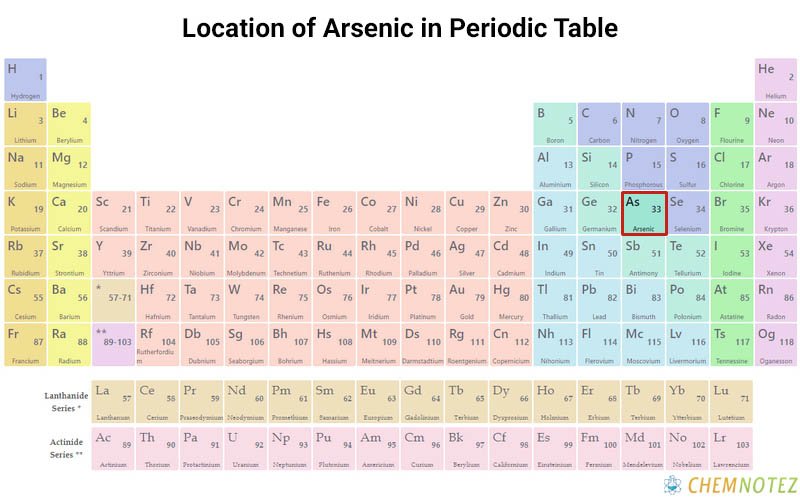
Lewis Dot Structure of Arsenic
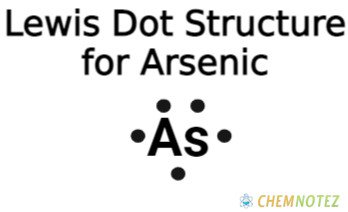
Bohr’s Atomic Model of Arsenic
Atomic Data of Arsenic
Physical Properties of Arsenic
| Color | Silvery-grey/Steel gray |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 74.922 |
| Weight | 74.921595 |
| Density | 5.75 |
| Atomic Radius | 1.85 |
| Ionization Energy | 947 kJ.mol -1 |
| Covalent Radius | 1.21A˙ |
| Ionic Radius | 0.222 nm (-2) 0,047 nm (+5) 0,058 (+3) |
| Electronic Gain Enthalpy | 77.574 kJ mol−1 |
| Electron Negativity | 2.18 |
| Electron Affinity | 77.574 kJ mol−1 |
| Melting Point | Sublimes at 616°C, 1141°F, 889 K |
| Boiling Point | Sublimes at 616°C, 1141°F, 889 K |
Chemical Properties of Arsenic
| Atomic Number | 33 |
| Group | 15 |
| Period | 4 |
| Block | p |
| Electronic Configuration | [Ar] 3d104s24p3 |
| Combustion | Non-combustible |
| Chemical Reactivity | Less reactive |
| Valency of Element | 5 |
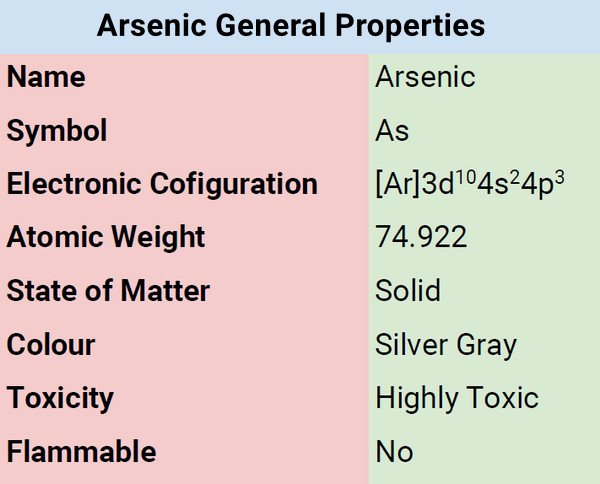
Different States of Arsenic
Arsenic is solid and appears in three allotropic forms namely black, yellow, and grey. Arsenic in a stable state is brittle crystalline and characterized by silver-gray color. When exposed to air, it tarnishes. When burn at high temperatures, it forms a white cloud of arsenic trioxide.


Uses of Arsenic
- One of the main uses of arsenic is poison. It is used to poison rats. Some arsenic compounds are used as insecticides, but their use is strictly controlled.
- Although it is known as a poisonous substance, arsenic does have medicinal applications too. organoarsenic compounds are used in the poultry industry, specifically added to poultry feed to prevent some types of poultry-related diseases. It is also used to improve weight gain.
- It also serves as a doping agent in semiconductors. A perfect example is gallium arsenide used in solid-state devices.
- Arsenic is also used in pyrotechnics, bronzing, and hardening shot.
- Some arsenic compounds are used to create special glass.
- The toxicity of arsenic is useful in preserving wood.
- Arsenic trioxide is one of the components used to treat some forms of cancer for over 500 years, specifically useful in the treatment of acute promyelocytic leukemia.
- Arsenic plays a vital role in lead alloying.
- A small percentage of arsenic is used in lead components of car batteries giving the battery more strength and durability.
- Gallium arsenide functions as a semiconductor material in integrated circuits.
- Arsenic is used to reduce the dezincification of brass.
Price of Arsenic
The cost of arsenic is dependent on supply and demand. Pure arsenic costs around $320 per 100 grams.
Interesting facts about Arsenic
- Don’t you know that arsenic comes from the word “zarnikh”, which is a Persian word for yellow orpiment?
- Arsenic is related to the Green word “arsenikos”, which means “masculine.”
- In some pyrotechnics, arsenic is added to create bluish-colored flame.
- If you bring arsenic or any materials containing arsenic to heat, it will release a distinct odor that is similar to that of garlic.
- Don’t you know that arsenic was used by soldiers during World War I?
- Arsenic ranked 53rd in the list of chemical elements found in the earth’s crust.
- Arsenic poisoning can take place and it happens because of exalted arsenic levels. A situation leading to arsenic poisoning includes drinking contaminated water.
- Although arsenic is known for its poisonous effect, it has medicinal importance too. A minute amount of arsenic is vital for animals’ health.
- Don’t you know that arsenic does not melt under usual pressure? It directly sublimes into gas. To bring arsenic to melt, you will need an extremely high temperature.
Frequently Asked Questions
Q1. How poisonous is arsenic?
Arsenic is extremely poisonous. If you don’t have sufficient knowledge about handling poisonous substances like arsenic, then you should not deal with it in any way. In fact, many arsenic compounds are poisonous too. it can kill a person.
In fact, in ancient times, arsenic and its compounds are purposely used to assassinate a person. Exposure to a small amount of arsenic for a long period of time can be detrimental to your health. Industries that are involved in the production or use of arsenic must stick to rules and guidelines for proper toxic handling.
Q2. What element destroys arsenic?
Unfortunately, it can’t be destroyed in the environment. What it does is it changes its form or attaches to separate from particles. It does so by reacting with oxygen and other molecules present in its surroundings such as in air, soil, or water.
Q3. What makes arsenic poisonous?
Arsenic is extremely poisonous in the sense that it is a very minute particle that can easily penetrate through the cells. Once it got into the cells, it can immediately cause injury and even death. Another thing that makes arsenic poisonous is its ability to directly interact with red blood cell membranes.
Q4. Does arsenic expire?
As alarming as it may seem, arsenic won’t go off the shelf. It has the ability to retain its poisonous effect no matter how long you store it.
Q5. Can you be immune to arsenic?
Arsenic is called the king of poison because there is no such way for you to develop your immunity to it. exposure to it or worst taking it in won’t make you immune to it. it will just end up killing you, something that is not worth the risk.
References
- https://www.thoughtco.com/interesting-arsenic-element-facts-603360
- https://www.rsc.org/periodic-table/element/33/arsenic
- https://www.livescience.com/29522-arsenic.html
- https://www.lenntech.com/periodic/elements/as.htm
- https://www.echemi.com/cms/557462.html
- https://www.worldofchemicals.com/587/chemistry-articles/few-interesting-things-about-arsenic-you-didnt-know.html
- https://www.ducksters.com/science/chemistry/arsenic.php
- https://umaine.edu/arsenic/what-is-arsenic/
- https://www.greenfacts.org/en/arsenic/l-2/arsenic-1.htm
- https://www.chemicool.com/elements/arsenic.html