What is Beryllium?
Beryllium is a chemical element, which formerly known by the name glucinium. It was derived from the Greek word “glykys”, which means “sweet” because of its characteristics.

Although it is sweet, it cannot be ingested because it is highly toxic. There are specific standards and work codes to follow to ensure safety in handling beryllium.
Although extreme caution is required when handling Beryllium, it is still widely used in many industries due to its unique properties. It is one of the lightest and most elastic metals. It is non-magnetic and highly resistant to nitric acid.
Where is Beryllium obtained?
It naturally occurs in the environment, such as the air, water, soil, and the earth’s crust. In fact, people are exposed to a very minute amount of beryllium through such activities as consuming food grown in soil, drinking water, and simply breathing the air.

Today, beryllium is obtained from bertrandite and beryl through a chemical process such as electrolysis. Various substances are mixed to obtain beryllium, such as molten beryllium chloride and sodium chloride.
History of Beryllium?
In 1798, Beryllium metal was discovered by Nicolas-Louis Vauquelin, a French chemist. He discovered beryllium in minerals like emerald and beryl, which during ancient times were used as gemstones.
In 1828, the element was successfully isolated by a French chemist, Antoine A.B. Bussy, and a German chemist, Friedrich Wohler. They were successful in extracting pure beryllium from beryllium chloride.
Classification, Properties and Characteristics
Beryllium is a soft metal characterized by its light silvery-gray color. It is strong but brittle. It’s non-magnetic but a very good thermal and electrical conductor. It is solid at room temperature and pressure. It can effectively transfer heat from hot to cold objects.
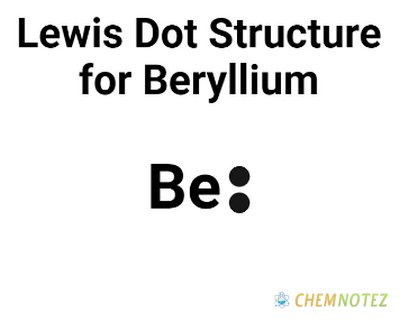
Lewis Dot Structure of Beryllium
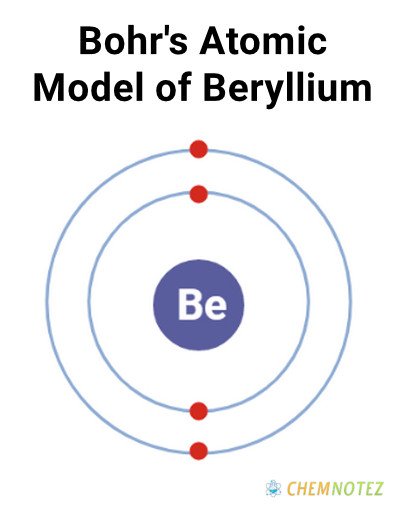
Bohr’s Atomic Model of Beryllium
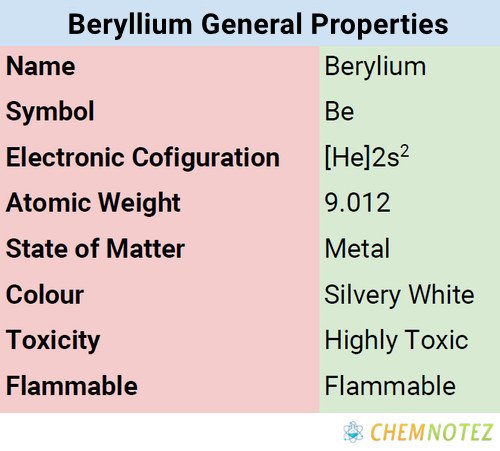
Atomic Data of Beryllium
Physical Properties of Beryllium
| color | silvery white/light silver-gray |
| odor | odorless |
| Taste | sweet |
| Atomic mass | 4.003 |
| Weight | 4.002602 |
| Density | 0.1785 gram/litre |
| Atomic Radius | 140 pm |
| Covalent Radius | 28 pm |
| Ionic Radius | 93 |
| Ionization Energy | 24.5874 eV |
| Electronic Gain Enthalapy | 48 |
| Electron Negativity | None |
| Electron Affinity | -0.5(2) |
| Melting Point | -272.2 °C |
| Boiling Point | −268.9 °C (−452 °F) |
Chemical Properties of Beryllium
| Atomic Number | 4 |
| Group | 2 Alkaline Earth Metal |
| Period | 2 |
| Block | s |
| Electronic configuration | [He]2s2 |
| Combustion | Combustible/will ignite if the surrounding is in fire |
| Chemical Reactivity | Relatively unreactive at room temperature |
| Valency of Element | +2 |
Different States of Beryllium
- Beryllium is an alkaline earth metal and remains solid at room temperature.
- Like any other metal, it is not reactive at room temperature, especially in its massive form.
- In fact, even at red heat, it remains non-reactive to water or steam.
- It does not oxidize in the air, specifically at temperatures below 600 °C.
- On the other hand, it can precipitate as ammonium phosphate with the help of acetoanilide.
What are the common uses of beryllium?
There are many uses for beryllium in today’s age, but some of the most noticeable are the following:
Used in alloys along with other substances
- Beryllium is combined with other substances such as alloys with nickel or copper to make electrical contacts, springs, gyroscopes, non-sparking tools, and spot-welding electrodes.
- The purpose of mixing beryllium with these substances is to increase thermal and electrical conductivity.
- Some are used as structural materials for making missiles, high-speed aircraft, communication satellites, and spacecraft.
Used in x-ray
- Beryllium is transparent to x-rays. That’s why it is used in x-ray lithography.
- It is also used in radiation windows for x-ray tubes.
Used as nuclear reactors
- Beryllium is one of the important components of nuclear reactors, specifically as a moderator or reflector of neutrons.
Used for ceramic work and nuclear work
- Beryllium has a high melting point, making it effective in other applications such as creating ceramic and nuclear work.
Used in creating spark-proof tools
- Beryllium is mixed with copper to create an alloy for the creation of spark-proof tools.
Used in creation of other materials
- Some beryllium alloys are used in creating brake disks, windshields, and some parts of the structural components of space shuttles.
Used for acoustics
- Beryllium is useful in making high-frequency speaker drivers because of its high rigidity and low weight.
Used in making dental alloys
- Beryllium is also used in creating several dental alloys.
Price of Beryllium
Pure beryllium is expensive. In fact, 98% pure beryllium costs around $600 to $800 per pound. That is raw beryllium, which has not undergone any machining. Some machining techniques cause the scraping of expensive parts of beryllium. Thus, the reason why many industries prefer to buy pure beryllium.
Interesting facts about Beryllium
- Don’t you know that beryllium is six times stiffer than steel in terms of weight?
- It is one of the lightest elements.
- Don’t you know that although beryllium is an alkaline earth metal, it is non-magnetic?
- Don’t you know that only three countries in the world currently process beryllium ores? These are China, the United States, and Kazakhstan.
- Beryllium played a crucial role in the discovery of neutrons.
- Beryllium has a sweet taste, but you cannot eat it because it is toxic when ingested. A taste test is one of the standard tests performed by chemists during earlier times.
- The discovery of beryllium was only accidental. It was discovered while investigating beryls, a mineral available in various colors.
- It ranked number 44 in the list of the most abundant chemical elements found in the earth’s crust.
- Don’t you know that the original name of beryllium was Glyceynum, from the Greek word “glykis,” which means sweet? It was eventually changed to beryllium in 1957.
Pictures of Beryllium

Frequently Asked Questions
Q1. Does beryllium pose a danger to humans?
Although beryllium has many uses in various industries, it is potentially dangerous to humans. That is why special precautions are observed when handling beryllium. Direct contact with fumes or dust of beryllium, especially in huge amounts, can injure the exposed parts of the body, such as the skin and eyes. It is also carcinogenic.
Q2. What types of diseases or health conditions can be obtained from prolonged exposure to beryllium?
There are many health conditions that can be obtained from prolonged exposure to beryllium, such as CBD or Chronic Beryllium Disease, and lung cancer, which is usually common in people who work in industries that primarily rely on beryllium handling. That is why there are certain precautionary measures in the workplace to ensure workers’ safety.
Q3. What substances react with beryllium?
Beryllium is reactive to some chemicals at a given temperature. For instance, it reacts with oxygen at an extremely high temperature. It forms beryllium oxide.
Q4. What makes beryllium unique from the rest?
There are many chemical elements, but what sets beryllium apart from the rest is that it has the highest melting point of all elements in the light metal group. Other notable characteristics unique to beryllium are its non-magnetic property, good thermal conductivity, and ability to resist concentrated nitric acid at a regular temperature. It also has the ability to resist oxidation when exposed to air.
Q5. What is the downside of beryllium?
Although beryllium has plenty of good qualities, it does have weaknesses, one of which is its toxicity, especially its fumes, dust, and soluble salts. It is brittle too, which increases the hazard associated with beryllium toxicity.
References
- https://en.wikipedia.org/wiki/Beryllium
- https://www.rsc.org/periodic-table/element/4/beryllium
- https://www.britannica.com/science/beryllium
- https://www.lenntech.com/periodic/elements/be.htm
- https://pubchem.ncbi.nlm.nih.gov/element/Beryllium
- https://www.chemicool.com/elements/beryllium.html
- https://byjus.com/chemistry/beryllium/
- https://beryllium.com/about-beryllium
- https://www.livescience.com/28641-beryllium.html
- https://www.azom.com/article.aspx?ArticleID=9095