What is Cadmium?
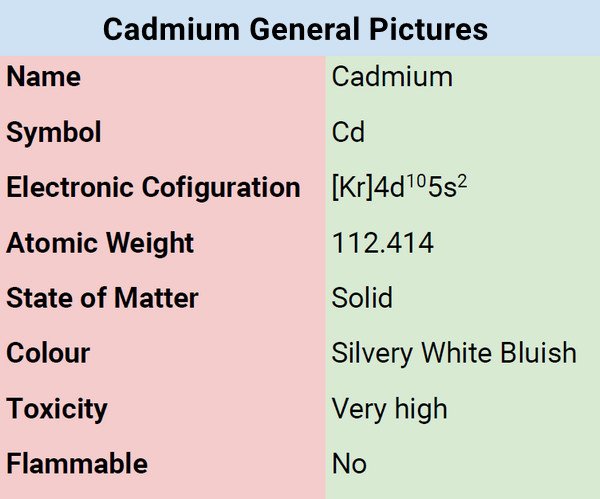
Cadmium is characterized by silvery-white, ductile metal characterized by a bluish tint when in pure form. It is a somewhat rare metal that is naturally present in the earth’s crust and ranks 67th in terms of abundance. Due to cadmium’s lack of taste and odor, chemical examination is frequently needed to determine its presence. It rarely occurs in pure form because it reacts so easily with other substances; instead, it is combined with other chemical structures to generate a range of compounds. Some of which are highly hazardous.
Where is Cadmium obtained?
Cadmium is present in lead and copper ores but is more commonly found in ores that contain zinc. Cadmium sulfide, is cadmium’s main mineral form. Cadmium is extremely uncommon in the Earth’s crust. Some forms of minerals like cadmium carbonate and otavite occur but extremely rare. Through natural phenomena like erosion or the burning of ores that contain cadmium in volcanic eruptions, cadmium can be dispersed in the air by the wind. Cadmium particles from the air fall to the land and into streams.
Although dissolved cadmium can occasionally be found in surface waters, its quantities are typically modest because it is easily assimilated by marine life, particularly shellfish.Industrial activities like mining, refining, and smelting metal ores, especially copper, lead, and zinc, play a substantial role in producing concentrated amounts of cadmium and dispersing it into the surrounding.
Additionally, the burning of fossil fuels, the burning of garbage, and the manufacture of steel all release cadmium into the air. Higher levels of cadmium may be present in the land and water close to industrial regions or waste dumps. According to estimates, human activities result in the discharge of between 4,000 and 13,000 tons of cadmium into nature each year.
History of Cadmium
Friedrich Stromeyer (1776–1835), a German chemist, was the first to discover pure cadmium in 1817. In his laboratory, he put zinc carbonate samples into heat and noticed that their color changes at high temperatures. Stromeyer reasoned that since pure zinc carbonate will not change its color, some contaminant must have triggered the process. In order to find the impurity, he developed an experimental method, and this allowed him to separate the silver-blue metal.

Classification, Properties and Characteristics of Cadmium
Cadmium is a bluish-white, ductile, soft metal that can be easily sliced with a knife. It is a very good conductor of electricity, resistant to corrosion, and chemical attacks. Its chemical characteristics resemble zinc in a number of ways. While soluble in acids, it is not soluble in alkalis. In fact, it tarnishes in the air. When the metal is burned, it produces brown cadmium oxide.
Lewis Dot Structure of Cadmium

Bohr’s Atomic Model of Cadmium
Atomic Data of Cadmium
Physical Properties of Cadmium
| Color | Silvery/Bluish-white |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 112.414 |
| Weight | 112.414 |
| Density | 8.69 |
| Atomic Radius | 2.18 Å |
| Ionization Energy | 867.772 kJ mol−1 |
| Covalent Radius | 1.40 Å |
| Ionic Radius | 158 pm |
| Electronic Gain Enthalpy | Not Stable |
| Electron Negativity | 1.69 |
| Electron Affinity | Not Stable |
| Melting Point | 321 °C (610 °F) |
| Boiling Point | 765 °C (1,409 °F) |
Chemical Properties of Cadmium
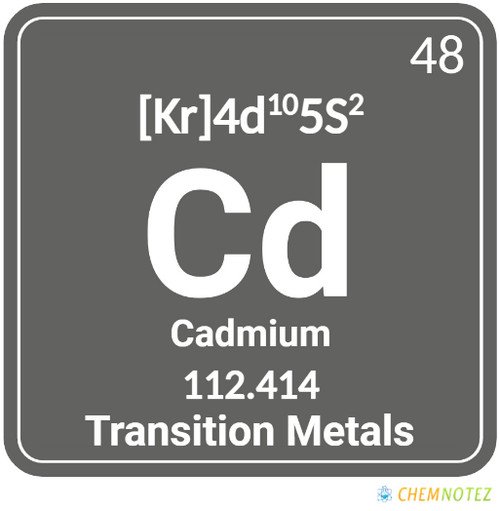
| Atomic Number | 48 |
| Group | 12 |
| Period | 5 |
| Block | d |
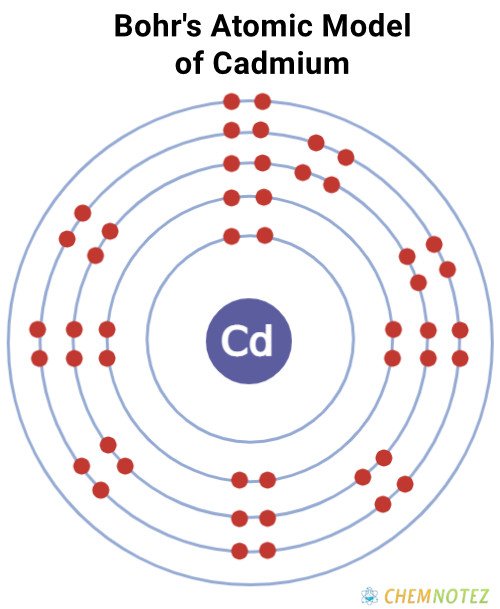
| Electronic Configuration | [Kr]4d105s2 |
| Combustion | Flammable but only in powder form |
| Chemical Reactivity | Highly Reactive |
| Valency of Element | +2 |
Different States of Cadmium
Cadmium belongs to the transition metal group, which means that it is solid at room temperature. It liquefies once the desired melting temperature is reached.

Uses of Cadmium
- Cadmium is mostly utilized in rechargeable batteries typically in combination with silver oxides and nickel. About three-quarters of cadmium in the United States is used for this reason.
- Copper, iron, steel, and brass are frequently electroplated with cadmium in situations where they will be exposed to corrosive substances or extreme weather conditions.
- Cadmium is essential to much cutting-edge technology, including solar cells.
- Cadmium is found in a number of other items and usually used to create unique solder alloys that melt at a significantly low temperature. It is occasionally used to stabilize some plastics.
- Some cadmium compounds are used in paints. Cadmium selenide provides colors that range from red to yellow, whereas cadmium sulfide gives paints a yellow tint.
- Because of its capacity to absorb released particles, cadmium may be used in nuclear reactors’ shields and control rods.
- Another application of cadmium is the production of phosphor compounds, which glow when exposed to electrons and are used in television tubes to display images.
- Cadmium is significant in molecular biology. It blocks voltage-dependent calcium channels from instability of calcium ions.
Price of Cadmium
The cost of cadmium fluctuates depending on supply and demand. Pure cadmium costs around $46 per 100 grams. The price is cheaper if you buy in bulk.
Interesting facts about Cadmium
- Don’t you know that there are around 0.1 parts per million of cadmium in the earth’s crust?
- Electroplating of cadmium is widely used in the aircraft industry.
- The leading producers of cadmium in the world are Germany, India, The Netherlands, Korea, the United States, Mexico, and China.
- A huge amount of cadmium in the body is carcinogenic (can cause cancer), specifically lung cancer.
- Cadmium was previously used in orange, yellow, and red paints because of its bright hue.
- Cadmium comes from the Latin word “cadmia” and the Greek word “kadmeia.
Frequently Asked Questions
Q1. How People Are Exposed to Cadmium?
Due to cadmium’s ability to be absorbed into the animal and plant foods that people eat, cadmium exposure occurs through nutrition. Cigarette smoke exposes people to greater cadmium exposure levels. People working in industries that primarily used cadmium are more prone to cadmium exposure, especially if caution is not observed. Cadmium is used or produced in industries like making batteries, soldering metal, or welding.
Q2. How does Cadmium Affects People’s Health?
Large doses of cadmium when consumed can induce severe stomach upset, nausea and vomiting, and diarrhea. High cadmium exposure affects people’s lungs and can be fatal. Exposure to low quantities of cadmium for a long period of time in the environment—through tobacco smoke, for example—increases the risk of renal disease and brittle bones. It is believed that cadmium causes cancer.
Q3. Is cadmium an essential element for life?
The metal cadmium is frequently found together with zinc in nature, and it has some functions that are comparable to those of zinc. Zinc is necessary for life, whereas some types of cadmium are poisonous.
Q4. How can you protect against cadmium?
You can protect yourself from the dangers caused by cadmium by giving up smoking. Cadmium in cigarette smoke can be ingested through the lungs. Avoid second-hand smoke ( inhalation of smoke from other people’s cigarettes). Consume organ meats and seafood in moderation as part of a healthy, balanced diet.
Q5. Can you burn cadmium?
Powdered cadmium burns and emits toxic and corrosive gases. Solid cadmium is non-flammable.
References
- https://sites.dartmouth.edu/toxmetal/more-metals/cadmium-an-illusive-presence/the-facts-on-cadmium/
- https://www.britannica.com/science/cadmium
- https://www.rsc.org/periodic-table/element/48/cadmium
- https://www.livescience.com/37044-cadmium.html
- https://www.lenntech.com/periodic/elements/cd.htm
- https://www.osha.gov/cadmium
- https://www.chemicool.com/elements/cadmium.html
- https://study.com/academy/lesson/cadmium-definition-facts-uses.html
- https://pubchem.ncbi.nlm.nih.gov/compound/Cadmium
- https://www.chemistrylearner.com/cadmium.html