What is Carbon?
Carbon is a nonmetallic element and one of the primary components of most life on Earth. It is more flexible than rubber and, in fact, stronger than steel. It is an incredible chemical element because it is widely distributed in nature, but ironically, not that plentiful.
As a matter of fact, it comprises only 0.025% of the earth’s crust. Although it can be considered scarce in nature, it has many uses and we are going to discuss everything carbon in this article.
Where is Carbon obtained?
There are many sources of carbon, such as the following:

- Diamonds – Don’t you know that diamonds are a chunk of carbon? If you left carbon in an extremely high pressure area, its atoms would be pushed together, forming a crystal called diamond.
- Pencil – Get a pencil and notice the black stuff. Yes, that’s actually a special type of carbon called graphite.
- Charcoal – Don’t you know that the primary ingredient of charcoal is carbon? Carbon compounds are a good source of energy and extremely good at holding onto heat.
- Petroleum products like gasoline – Carbon is one of the essential components of gasoline.
- Plastics – Things made from plastic materials have carbon in them.
- Plants – Carbon is one of the integral elements of plants. Without it, it is impossible for plants to exist.
History of Carbon
The term carbon is derived from carbo, a Latin word for “charcoal.” Smithson Tennant, an English chemist, demonstrated in 1797 that diamond is a form of carbon.Carbon has been known since ancient times, making it the sixth most abundant element in the universe. There are three naturally occurring carbon allotropes; diamond, graphite, and amorphous.
Classification, Properties and Characteristics of Carbon
Carbon is classified depending on its number of atoms. It can be grouped as primary, secondary, tertiary, and quaternary. However, the classification only applies to saturated carbons. Atomic carbons are short-lived and stabilized in various multi-atomic structures with plenty of allotropes. The three well-known carbon allotropes are amorphous, graphite, and carbon.
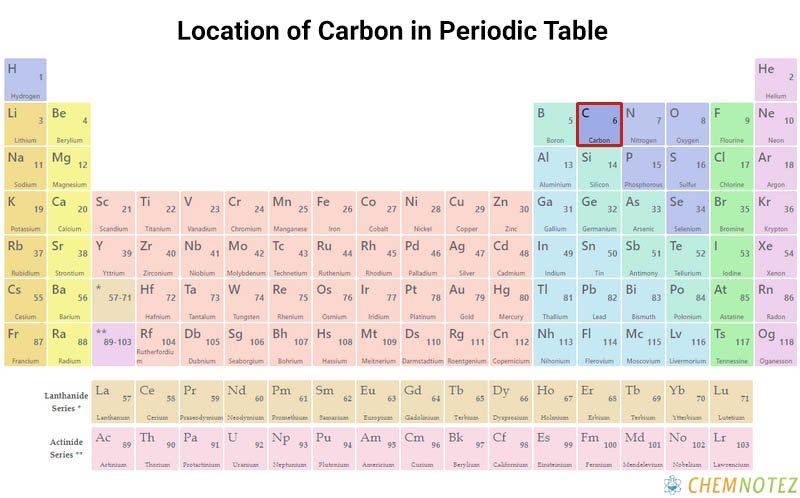
Lewis Dot Structure of Carbon
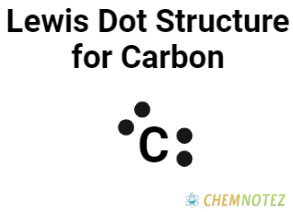
Bohr’s Atomic Model of Carbon
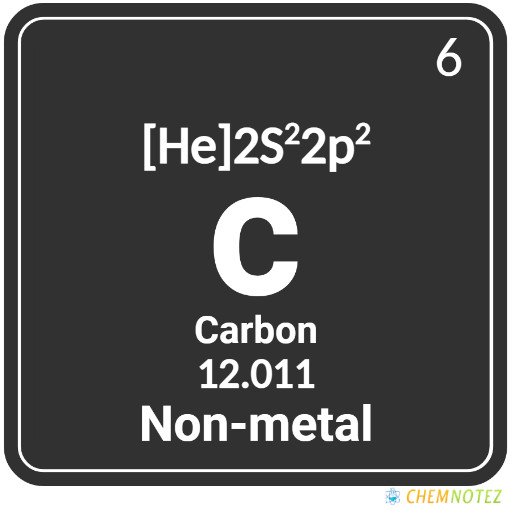
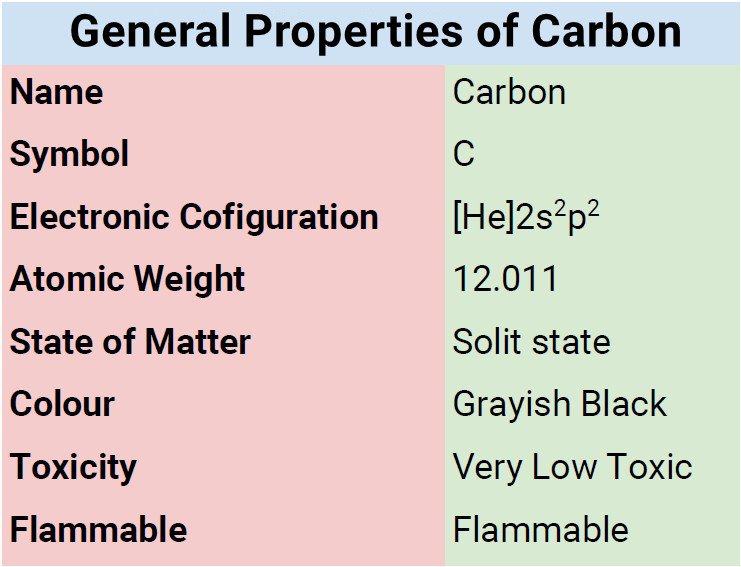
Atomic Data of Carbon
Physical Properties of Carbon
| Color | black |
| Odor | odorless |
| Taste | tasteless |
| Atomic Mass | 12.011 |
| Weight | 12.0096 to 12.0116 |
| Density | 2.2 g.cm-3 at 20°C |
| Atomic Radius | 70 pm |
| Ionization Energy | 11.260 eV |
| Covalent Radius | 76 pm |
| Ionic Radius | 0.26 nm (-4) ; 0.015 nm (+4) |
| Electronic Gain Enthalpy | 1086.1 kJ.mol -1 |
| Electron Negativity | 2.55 (Pauling Scale) |
| Electron Affinity | 1.263 eV |
| Melting Point | 3,550 °C (6,420 °F) |
| Boiling Point | 4,827 °C (8,721 °F) |
Chemical Properties of Carbon
| Atomic Number | 6 |
| Group | 14 |
| Period | 2 |
| Block | p |
| Electronic Configuration | 1s22s22p2 |
| Combustion | It is highly combustible |
| Chemical Reactivity | Pure carbon is reactive to other chemicals |
| Valency of Element | 4 |
Different States of Carbon
Carbon is classified as a non-metal and is solid at room temperature.
What are the common uses of carbon?
Although carbon is a common chemical element, it is not that plentiful. However, its uses are undeniable. It is a unique element because it has the ability to form strong bonded chains and be sealed off by hydrogen atoms. Some of the most notable uses of carbon are the following:
Feedstock
- A fraction of carbon is used in the petrochemical industries as a feedstock, such as solvents, plastics, paints, and fibers.
Metal smelting
- Carbon in impure forms such as coke (from coal) and charcoal (from wood) is one of the integral components in the metal industry, particularly in creating steel and iron.
Graphite
- Carbon in graphite form is used in industries like pencil making as well as making brushes to be used in electric motors and furnace linings.
Filtration and purification process
- Activated charcoal plays an important part in the filtration and purification process.
Carbon fiber
- This type of carbon is known for being lightweight and durable, which is why it is used in a variety of industries, including rockets and airplanes. It is also used for creating fishing rods, skis, and tennis rackets.
- You can find a huge amount of carbon in kitchen extractor hoods and respirators.
Industrial diamonds
- Carbons are used in the form of industrial diamonds, particularly in drilling and cutting rocks. In fact, films of diamond are used to protect surfaces like razor blades.
Price of Carbon
The price of carbon is dependent on the form it takes. The least expensive is the lowest carbon rank. For instance, sub-bituminous coal costs around 0.038 USD/kg carbon. The cost of synthetic industrial diamond commonly used for polishing and grinding ranges between $1200 and $13,300/kilogram. The cost of graphite flake is around 0.9 USD/kg carbon. Large synthetic diamonds commonly used for industrial purposes can cost a million dollars per kilogram.
Interesting facts about carbon
- Carbon is tagged as the duct tape of life. Every living thing has carbon. In fact, even some of the dead ones have carbon in them too. It is called the “duct tape of life” because it has the ability to bind atoms to one another.
- There is plenty of carbon in the universe. It ranked fourth in the list of the most abundant elements in the universe and 15th in the earth’s crust.
- Carbon loves to bond. It has the ability to form four bonds with other elements, leading to the formation of hundreds of thousands of compounds.
- Don’t you know that about 20% of the body is made up of carbon? It makes up life forms and some of the substances it makes, such as sugars and fats. It can form rings and chains through the process of catenation. Living things have carbon in them along with other elements like hydrogen, nitrogen, oxygen, and other elements.
- Carbon paper is widely used in school and office settings. It is the reason why the phrase “carbon copy” became popular.
- Carbon dioxide is a byproduct of the exhalation process.
- Carbon is used to find out the age of organic materials through the process of carbon dating.
Pictures of Carbon


Frequently Asked Questions
Q1. Is carbon more durable and stronger than steel?
We are well aware that steel is strong and durable, thus, the reason why it is commonly used in the construction industry. However, carbon fiber has been making a name in the construction world. It is also strong and durable, in fact, even stronger than steel.
Q2. What makes carbon such an incredible element?
Carbon is one of the most amazing chemical elements because it connects to a wide array of elements, thereby forming almost all forms of life. Carbon in charcoal form is known to primitive humans.
Q3. Why is carbon referred to as fossil fuels?
Carbon is called fossil fuel because some forms of it come from the chemical remains of prehistoric animals and plants. A perfect example is the fuel in the gas tank. Not to mention, all living things on earth have carbon, including us humans. In fact, there’s a lot of carbon in humans. For example, if you weigh 100 pounds, about 18 pounds of that weight is pure carbon. The same thing goes with plants and other living creatures. Although the amount of carbon in plants is higher than in humans.
Q4. What is a carbon cycle?
It is the process in which carbon is exchanged between various parts of the earth and the living organisms. The carbon cycle is essential to life on Earth, enabling carbon to continue reusing and recycling carbon.
Q5. Is there a dangerous form of carbon?
Yes, there is, and it is in the form of carbon monoxide. It is toxic to both humans and animals. Carbon monoxide takes place when there is no sufficient oxygen to form carbon dioxide. Carbon monoxide poisoning happens, and it can be very fatal.
References
- http://www.chem4kids.com/files/elements/006_speak.html
- https://en.wikipedia.org/wiki/Carbon
- https://www.britannica.com/science/carbon-chemical-element
- https://www.livescience.com/28698-facts-about-carbon.html
- https://www.rsc.org/periodic-table/element/6/carbon
- https://www.worldofchemicals.com/571/chemistry-articles/all-that-you-need-to-know-about-carbon-element.html
- https://www.lenntech.com/periodic/elements/c.htm
- https://www.chemicool.com/elements/carbon.html
- https://www.thoughtco.com/carbon-element-facts-606515
- https://www.theguardian.com/environment/2011/feb/03/carbon