What is Copper?
Copper is an element widely used at home. Its pure and chemical compounds are used in various applications. It comes from the Latin word “cuprum”, which means “from the isle of Cyprus.” The place is known for copper mines.
Where is Copper obtained?
The earth’s crust contains copper. The slow reactivity of copper is the reason why it is usually available in pure form. Today, copper is extracted from minerals like copper carbonates and copper sulfides. Copper is recyclable, and, in fact, a huge percentage of copper comes from recycling. Chile is the main producer of mined copper. It produces around 33% of mined copper in the world.
History of Copper
Copper has long been used in ancient times and at that time they use it as bronze. In Roman times, copper was mined in Cyprus. It was one of the first elements used by men. The artifacts of copper date back to 9000 BC. In ancient times, copper was used to making tools and decorative pieces because of its durability and malleability. It’s unfortunate that no one is named for the specific discovery of copper.

Classification, Properties, and Characteristics of Copper
Copper is a soft orange metal known as a good conductor of heat and electricity. It is ductile, which means that you can easily bend or stretch it. when compared with other elements, copper is not that reactive, but reacts gradually when gets in contact with water and air. When copper is exposed to air, the copper turns brown. When exposed to water, copper corrodes and forms verdigris (green carbonate). The green color of the Status of Liberty is all because of copper.

Lewis Dot Structure of Copper

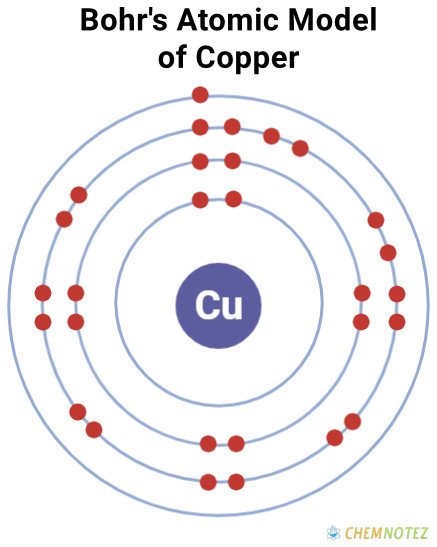
Bohr’s Atomic Model of Copper
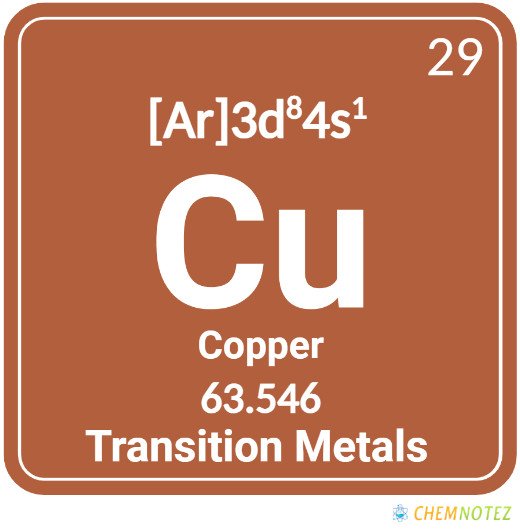
Atomic Data of Copper
Physical Properties of Copper
| Color | Shiny reddish/Orange red |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 63.546 g.mol -1 |
| Weight | 63.546 |
| Density | 8.96 at 20 °C (68 °F) |
| Atomic Radius | 1.96 Å |
| Ionization Energy | 745.482 kJ mol−1) |
| Covalent Radius | 1.22 Å |
| Ionic Radius | 0.096 nm (+1) ; 0.069 nm (+3) |
| Electronic Gain Enthalpy | 119.159 kJ mol−1 |
| Electron Negativity | 1.90 |
| Electron Affinity | 119.159 kJ mol−1 |
| Melting Point | 1,083 °C (1,981 °F) |
| Boiling Point | 2,567 °C (4,653 °F) |
Chemical Properties of Copper
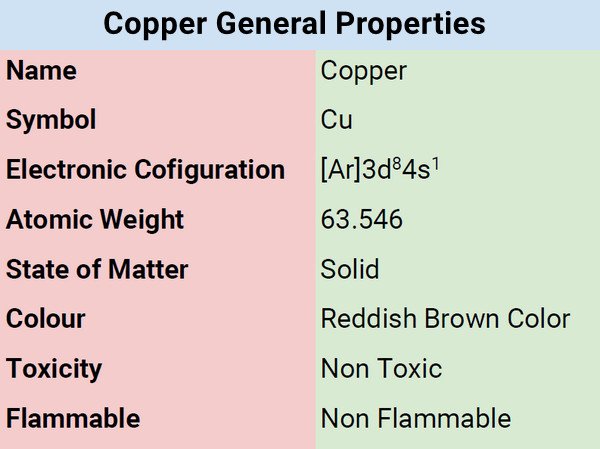
| Atomic Number | 29 |
| Group | 11 |
| Period | 4 |
| Block | d |
| Electronic Configuration | [Ar]3d104s1 or2-8-18-1 |
| Combustion | Does not burn itself |
| Chemical Reactivity | Low chemical reactivity |
| Valency of Element | 1 and 2 |
Different States of Copper
As a transition metal, copper is solid at room temperature. It exists in two oxidation states, specifically +1 and +2.



Uses of Copper
- Copper is one of the precious metals used to make coins including gold and silver.
- Copper is used in making gun metals.
- The majority of electrical equipment contains copper like motors and wiring. Thanks to copper’s ability to conduct heat and electricity.
- Copper is used in the construction industry specifically in the plumbing and roofing system.
- Copper is used in industrial machinery specifically as heat exchangers.
- Copper compounds like copper sulfate are used as an agricultural poison.
- Copper plays a vital role in water purification. It serves as an algicide.
- Fehling’s solution, a copper compound plays an important role in the chemical test. It is used to check for the presence of sugar.
- Copper has biological roles. At least 1.2 mg of copper is needed by a typical adult on a daily basis. Copper facilitates enzymes in transferring energy in cells.
- Crustaceans use copper complexes to transport oxygen around their bodies.
- Copper is usually used in the gutter, roofing, and rainspout because it does not corrode easily.
- Cookware and cooking utensils contain copper.
- Some important alloys like bronze and brass contain copper.
- Copper compounds like copper sulfate are effective fungicides. It is also used as an algicide in ponds, lakes, and rivers.
Price of Copper
The cost of copper is dependent on supply and demand. Pure copper costs around $10, but if you purchase in bulk, the cost would be around $1 per 100 grams.
Interesting facts about Copper
- Don’t you know that if you lay out all copper wiring in an average car, it would stretch to 1.5km?
- Don’t you know that coins from 1783 to 1837 were made from pure copper? Years after that, coins were made from bronze, which is 95% copper, and a 5% mixture of other materials like zinc and tin.
- Don’t you know that humans need copper in their diet? A trace amount of copper in the body plays an essential role in the formation of red blood cells.
- Some food items are a great source of copper such as potatoes, beans, grains, and leafy vegetables.
- An extremely high level of copper can cause a variety of health problems such as vomiting, abdominal pain, and jaundice or yellowish discoloration of the skin.
- If you consumed a high level of copper for a long period of time, you will suffer from convulsions, diarrhea, and anemia.
- Copper is used in making antimicrobial garments such as antimicrobial socks because of its antimicrobial property. It can fight off bacteria, viruses, and yeast.
- Some intrauterine devices, a birth control method, contain copper. Copper wirings attached to IUD have the ability to kill sperm and eggs thereby preventing pregnancy.
- Don’t you know that copper is one of the rare metals that does not have silver or gray color?
- Don’t you know that the largest single piece of copper weighed 520 tons?
- Don’t you know that the majority of copper ore mined today only has 1% copper?
- Don’t you know that Egyptians used the symbol of ankh to represent copper?
- Don’t you know that copper turns black when heated in a burner? It is a result of the copper metal reacting with oxygen.
Frequently Asked Questions Copper
Q1. Does copper have effects on humans?
The effects of copper on humans depend on the amount of copper and frequency of exposure. Exposure to a high level of copper for a long period of time can cause eye, nose, and throat irritation. It causes vomiting, stomach aches, headaches, and diarrhea. If you absorb a high level of copper, you will be in a serious condition. It could damage your kidneys and liver. If not addressed right away could lead to death.
Q2. What is special about copper?
Copper is special in the sense that it is an element and mineral vital for everyday life. it is widely used in the industrial sector because of its malleability, ductility, and ability to transfer heat and energy. Not to mention, copper also has the ability to resist corrosion.
Q3. Can you live without copper?
A trace amount of copper is essential to human health. copper together with fatty acids and amino acids is needed in regulating metabolic processes.
Q4. Is copper good for the heart?
Copper, although vital in some bodily processes, an extremely high level of copper can be dangerous to the heart. It increases the incidence of heart failure.
Q5. What does copper chemically react with?
If you bring copper to heat, it becomes reactive to oxygen and forms black copper oxide. This copper compound reacts with hydrogen gas and forms copper metal and water.
References
- https://www.thoughtco.com/interesting-copper-element-facts-603357
- https://www.britannica.com/science/copper
- https://www.livescience.com/29377-copper.html
- https://www.rsc.org/periodic-table/element/29/copper
- https://www.lenntech.com/periodic/elements/cu.htm
- https://www.ducksters.com/science/chemistry/copper2.php
- https://www.chemicool.com/elements/copper.html
- https://byjus.com/chemistry/copper/
- https://sciencenotes.org/copper-facts/
- http://chemed.chem.purdue.edu/demos/main_pages/9.9.html