What is Gallium?
Gallium is a blue-gray metal and its structure is described as orthorhombic crystalline. When in pure form, gallium is silvery, soft, in fact, too soft that it can be cut using a knife. It is stable in water and air but has the ability to dissolve alkalis and acids.
Where is Gallium obtained?
Gallium is not available in free elements and in any minerals. However, it is available in trace amounts of some compounds like bauxite and zinc ores. Gallium is obtained by smelting. Most commercially produced gallium is extracted from the byproduct of zinc and aluminum. Russia, Germany, France, and Australia are the biggest producers of gallium.

History of Gallium
The discovery of gallium is linked to Paul-Émile Lecoq de Boisbaudran in Paris in 1875. He was able to notice a violet line in the atomic spectrum of some zinc. He knew at that time that it contains an unknown element. However, at that time he didn’t realize that such properties and existence were already predicted by Mendeleev. In November of 1875, he was able to isolate and purify this new metal and realized that it was like aluminum. He eventually announced it to the French Academy of Sciences in December of 1875.

Classification, Properties, and Characteristics of Gallium
Gallium has the typical characteristic of the metal. However, it has one of the longest temperature ranges in the liquid of any metal, and even at high temperatures, it still has a low vapor pressure. It has a strong possibility to supercool below its freezing point. To trigger solidification, gallium would require seeding. It is brittle and a poor conductor of electricity.
Lewis Dot Structure of Gallium

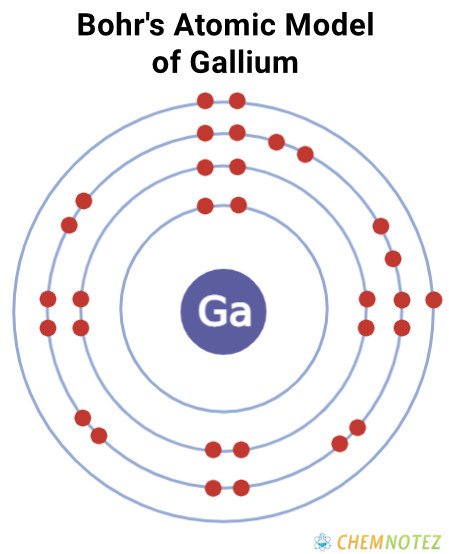
Bohr’s Atomic Model of Gallium
Atomic Data of Gallium
Physical Properties of Gallium
| Color | Silvery White/Silvery blue |
| Odor | Odorless |
| Taste | Metallic taste |
| Atomic Mass | 69.72 g.mol -1 |
| Weight | 69.723 |
| Density | 5.1 g.cm-3 at 20°C |
| Atomic Radius | 135 pm |
| Ionization Energy | 578.8 kJ/mol |
| Covalent Radius | 1.23 Å |
| Ionic Radius | 0.083 nm (+3) |
| Electronic Gain Enthalpy | 41.49 kJ mol−1 |
| Electron Negativity | 1.81 |
| Electron Affinity | 41.49 kJ mol−1 |
| Melting Point | 29.78 °C (85.6 °F) |
| Boiling Point | 2,403 °C (4,357 °F) |
Chemical Properties of Gallium
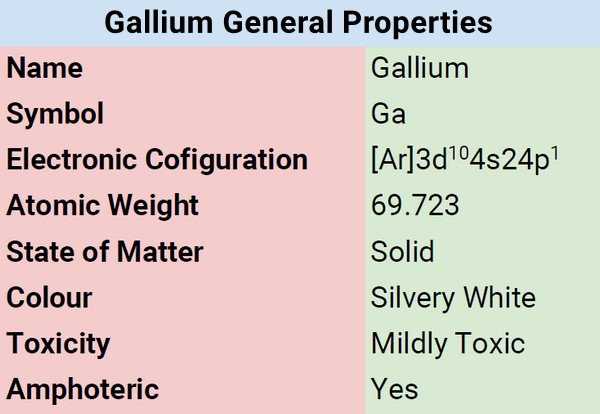
| Atomic Number | 31 |
| Group | 13 |
| Period | 4 |
| Block | p |
| Electronic Configuration | [Ar]3d104s24p1 |
| Combustion | Non-combustible |
| Chemical Reactivity | Highly Reactive |
| Valency of Element | 3 |
Different States of Gallium
Gallium is somewhat reactive and would readily react with alkalis and acids. Its oxidation state is +3. When it freezes or solidifies, it expands by 3.1%. The reaction is somewhat the same as when you put water in the freezer to turn it into ice. You should not store gallium in glass or metal containers because of this particular reaction.


Uses of Gallium
- The primary use of gallium is in the electronics industry. About 95% of produced gallium is used to make gallium arsenide. It is a compound used to make infrared circuits, blue and violet LEDs, semiconductors, and microwaves.
- A particular gallium compound, gallium arsenide, produces laser light from electricity. Thus, the reason why it is used in making solar panels.
- Another gallium compound, gallium nitride serves as a semiconductor in Blu-ray technology, pressure sensors (touch switches), and mobile phones.
- It is used to make low-melting alloys because of its ability to bond easily.
- It is used to make high-temperature thermometers, cooling and heating devices, heat transfer systems, and barometers.
- Some pharmaceuticals and radiopharmaceuticals used gallium. A perfect example is the radioactive isotope Ga-67 used in nuclear medicine tests. Its purpose is to look for inflammation and infection, most of all, it is used to detect cancer cells in the body.
- Gallium nitrate is one of the treatments for hypercalcemia, a medical condition that if not treated can lead to the growth of bone tumors.
- Gallium arsenide is used to substitute silicon because the two somewhat share the same structure. It is used in red light-emitting diodes (LEDs) because it has the ability to convert electricity to light.
Price of Gallium
The cost of gallium is dependent on supply and demand. Pure gallium costs around $220 per 100 grams. The price is cheaper when buying in bulk.
Interesting facts about Gallium
- Of all metals, gallium has the largest liquid range.
- You cannot keep liquid gallium in a glass or metal container because of its ability to expand when freezes.
- A large amount of gallium trichloride is used by Neutrino Observatory, in Italy to study solar neutrinos produced in the sun.
- When gallium is in its pure form, it creates a vibrant silver color. It turns blue-gray when in solid form.
- Gallium is used to stabilize crystal structures in nuclear bombs.
- Gallium turns into a lustrous mirror when used to paint glass.
- Don’t you know that the boiling point of gallium is eight times higher than the melting point?
- Don’t you know that gallium is named after France, which is the home country of the person responsible for discovering gallium, Paul-Emile Lecoq de Boisbaudran? Gaul is the older name for France.
- Don’t you know that gallium is one of the few elements that has a melting point near room temperature?
- Don’t you know that liquid gallium has the ability to wet the skin, glass, and porcelain? When gets in contact with glass, it forms a reflective surface similar to that of the mirror.
Frequently Asked Questions
Q1. What is gallium considered a rare element?
Gallium is one of the rarest elements on earth. In the continental crust, gallium content is only 19 ppm. It is rare and special at the same time because it is not available in elemental form. It is only available in bound forms, such as in the ores of zinc, aluminum, and germanium.
Q2. What happens if you touch the gallium?
You have to be cautious when touching gallium. It is a highly corrosive chemical. Once gets in contact with the skin, it causes a burning and irritating reaction. It does not only damage the skin, but also the eyes. If you breathe in gallium, it can irritate the nose and throat, thus, the reason why you cough and wheeze. Exposure to gallium for a long period of time can damage the kidneys and liver.
Q3. Is gallium toxic to eat?
Gallium is not that harmful in a minute amount. However, you should not purposely eat it. eating it can be potentially fatal.
Q4. Does gallium break easily?
At room temperature, gallium is soft, brittle, and breaks easily. So, does gallium break easily? The answer is yes!
Q5. Can gallium be used as a weapon?
Yes. A particular compound of gallium is used as a nuclear weapon pit. It is a nuclear weapon component that triggers a fission chain reaction. The gallium compound responsible for this reaction is plutonium-gallium alloy.
References
- https://www.britannica.com/science/gallium
- https://www.livescience.com/29476-gallium.html
- https://www.lenntech.com/periodic/elements/ga.htm
- https://www.rsc.org/periodic-table/element/31/gallium
- https://www.thoughtco.com/gallium-facts-606537
- https://sciencenotes.org/gallium-element-facts/
- https://www.chemicool.com/elements/gallium.html
- https://www.ducksters.com/science/chemistry/gallium.php
- https://study.com/academy/lesson/gallium-uses-facts.html
- https://www.vedantu.com/evs/interesting-facts-about-gallium