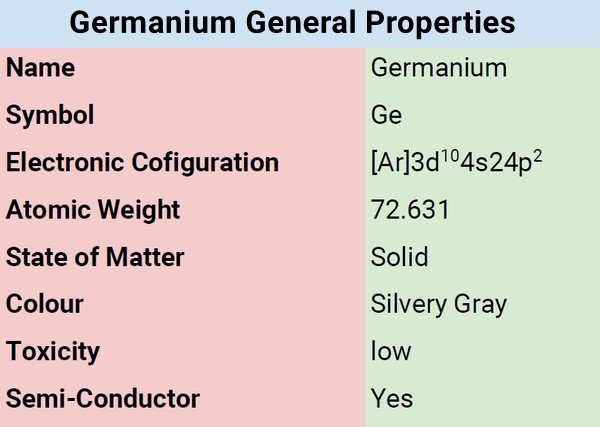
What is Germanium?
Germanium is a silvery gray chemical element that belongs to the group called metalloids. It’s between the silicon and tin groups. As a metalloid, its properties are somewhat metals and nonmetals, which makes it all the more intriguing and interesting.
Where is Germanium obtained?
It is rare to find germanium ore, and when found, usually in minute quantities. You can also find germanium minerals in zinc ores. Commercially produced germanium involves processing zinc smelter flue dust, which is a by-product of coal combustion.

History of Germanium
The discovery of germanium took place in 1886 and was linked to Clemens A. Winkler of Freiberg, Germany. A miner working in the HImmelsfurst silver mine discovered an unusual ore in September 1885. To check for the qualities of that particular unusual ore, it was sent to a nearby mining academy and they certified that it was indeed a new mineral. It was further found out that the ore contains silver (75%), and sulfur (18%), and the remaining 7% is unknown. It took almost a year to realize that it was indeed a new element.

Classification, Properties, and Characteristics of Germanium
At room temperature, germanium is hard, and shiny, with silver-gray color. Although it is solid, it is very brittle. If you put it inside the freezer or when solidify, it expands. It is linked to its metalloid properties. Germanium is also a semiconductor, thus, the reason why it is mainly used in the electronic industry. At normal temperatures, germanium is not reactive to oxygen, but when exposed to high temperatures, it forms germanium dioxide.
Lewis Dot Structure of Germanium

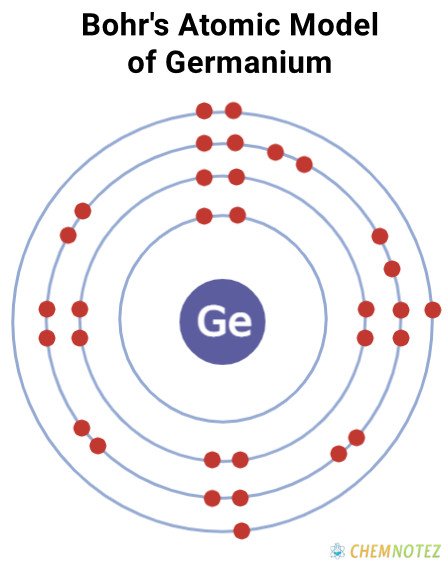
Bohr’s Atomic Model of Germanium
Atomic Data of Germanium

Physical Properties of Germanium
| Color | Silvery white/Gray white |
| Odor | Odorless |
| Taste | Bitter |
| Atomic Mass | 72.630 |
| Weight | 72.630 |
| Density | 5.3234 |
| Atomic Radius | 2.11 Å |
| Ionization Energy | 762.179 kJ mol−1 |
| Covalent Radius | 1.20 Å |
| Ionic Radius | 0.093 nm (+2) ; 0.054 nm (+4) |
| Electronic Gain Enthalpy | 118.939 kJ mol−1 |
| Electron Negativity | 2.01 |
| Electron Affinity | 118.939 kJ mol−1 |
| Melting Point | 938.25°C, 1720.85°F, 1211.4 K |
| Boiling Point | 2833°C, 5131°F, 3106 K |
Chemical Properties of Germanium
| Atomic Number | 32 |
| Group | 14 |
| Period | 4 |
| Block | P |
| Electronic Configuration | [Ar] 3d104s24p2 |
| Combustion | Flammable |
| Chemical Reactivity | Reactive |
| Valency of Element | 4 |
Different States of Germanium
Germanium is solid at room temperature. It forms a stable oxidation state, specifically +2 and +4. Germanium compounds are more stable, although most of their oxidation states are dependent on their classification as a metalloid.

Uses of Germanium?
- As a semiconductor, germanium is used as a transistor in various electronic applications.
- The high index dispersion and refraction of germanium oxide are the reasons why it is used in wide-angle camera lenses as well as objective lenses in microscopes.
- Germanium serves as an alloying agent and as a catalyst in fluorescent lamps.
- Germanium and its compound, specifically germanium oxide remains transparent to infrared radiation, thus, the reason it is used in infrared spectroscopes and infrared optics.
- Germanium is combined with silicon to create silicon germanium to be used in electronics, some metal alloys, and solar cells.
- It is used in transistors.
- Some germanium compounds are toxic to pathogens but safe for mammals.
- Some germanium compounds are used in the healthcare industry because of their ability to stop certain bacteria’s activities. Thus, the reason why germanium compounds are used as chemotherapeutic agents.
Price of Germanium
The cost of germanium is dependent on supply and demand. It is more expensive when compared to other elements. Pure germanium costs around $360 per 100 grams. If you purchase in bulk, the price is around $120 per 100 grams.
Interesting facts about Germanium
- Don’t you know that germanium is one of the few elements that expand once freezes?
- Geranium shares the same characteristics as silicon, gallium, antimony, and bismuth.
- Don’t you know that germanium comes from Germany’s Latin name?
- The earth’s crust has around 1.5 parts per million germanium by weight.
- The amount of germanium in the solar system is around 200 parts per billion in terms of weight.
- The significance of germanium was recognized during World War II when germanium was used in high-resolution radar receivers. It paved the way for the invention of the very first germanium transistor.
- Don’t you know that germanium was first named ekasilicon by Dmitri Mendeleev? Winkler eventually changed the name to Neptunium.
- The majority of germanium production today is from China.
- Don’t you know that the very first voyager space exploration mission that took place in 1970 primarily relied on the power produced by silicon-germanium photovoltaic cells?
Frequently Asked Questions
Q1. Why is germanium unique from the rest?
Germanium is distinct from the rest in the sense that it is a semiconductor, transparent to infrared radiation, and has the ability to expand when it freezes.
Q2. Is germanium safe for humans?
Although the biological significance of germanium has not yet been established up to this date, it is worth knowing that its toxicity is relatively low. However, there were reported cases of renal failure and death linked with the intake of germanium products. That is why it is important to handle germanium properly and make sure that it is not ingested in any way.
Q3. Is germanium affected by water?
Germanium is not reactive to water, although it is feasible thermodynamically speaking.
Q4. Is germanium good for the skin?
It has been found that germanium in the right amount is good for the skin. It can improve rough skin. Hence, the very reason why it is one of the components of cosmetics and other skin care products like bath salts. However, the one that is used for cosmetic and skin care purposes is water-soluble organic germanium.
Q5. Is germanium good for the heart?
Although there are safety concerns about the use, and specific ingestion of germanium, it is used to manage some medical conditions such as heart and blood vessel conditions. Examples are high cholesterol, high blood pressure, and cardiac-related conditions.
It is also sometimes used to treat eye-related conditions like cataracts and glaucoma. It is also significant in the treatment and management of cirrhosis and hepatitis.
References
- https://www.rsc.org/periodic-table/element/32/germanium
- https://www.britannica.com/science/germanium
- https://www.livescience.com/29520-germanium.html
- https://www.ducksters.com/science/chemistry/germanium.php
- https://www.lenntech.com/periodic/elements/ge.htm
- https://www.chemicool.com/elements/germanium.html
- https://www.thoughtco.com/germanium-facts-606538
- https://www.echemi.com/cms/498273.html
- https://en.wikipedia.org/wiki/Germanium
- https://pubchem.ncbi.nlm.nih.gov/element/Germanium