What is Hydrogen?
Hydrogen is a gaseous substance that is odorless, tasteless, and colorless. It is the most abundant element in the universe, but it only makes up 0.14% of the Earth’s crust (weight). A vast quantity of hydrogen can be found in the atmosphere, oceans, lakes, rivers, and ice packs.
Hydrogen can also be found in petroleum, animal, and vegetable tissue as an innumerable carbon compound. Under normal conditions, hydrogen is a loose aggregation of hydrogen molecules.
However, hydrogen is most commonly known for its chemical characteristic of burning with oxygen to form water. Thus, the Greek words “hydro” and “genes”, which means “maker of water.”
Presence or where Hydrogen is obtained
Hydrogen can be obtained from various sources. The major producers of hydrogen are fossil fuels, particularly natural gas. Other valuable sources of hydrogen are the following:
- Solar – The energy needed to produce hydrogen can be sourced from sunlight. Although sunlight is abundant, it is only available for a few hours a day. There are various processes used to produce hydrogen using solar such as electrolysis, photoelectrochemical, solar thermochemical hydrogen, and photobiological.
- Biomass – It is an abundant renewable source and can be converted to hydrogen and hydrogen byproducts. The processes used to produce hydrogen using biomass include microbial biomass conversion, biomass-derived liquid reforming, and biomass gasification.
- Wind – Wind plays a vital role in generating electricity, which can then be used for water electrolysis to produce hydrogen. The hydrogen product is then used to fuel vehicles.
- Plants and sugars – There is abundant hydrogen in plants and sugars generated through the process of photosynthesis in plants.
History of Hydrogen?
Hydrogen was discovered in 1766 by an English scientist named Henry Cavendish. He conducted an experiment using hydrochloric acid and zinc. During his experiment, he accidentally discovered hydrogen and noticed that it produced water when burned.
Although, Cavendish was the one who discovered hydrogen, the one who named it hydrogen was Antoine Lavoisier, a French chemist.

Classification, Properties and Characteristics of Hydrogen
Hydrogen is a gas of diatomic molecules (H2). Hydrogen is an odorless, colorless, and tasteless gas under standard conditions. However, it is highly flammable and burns with an invisible flame. In other words, when it comes into contact with oxygen, it burns.
Physical Properties of Hydrogen
| color | colorless |
| odor | odorless |
| Taste | tasteless |
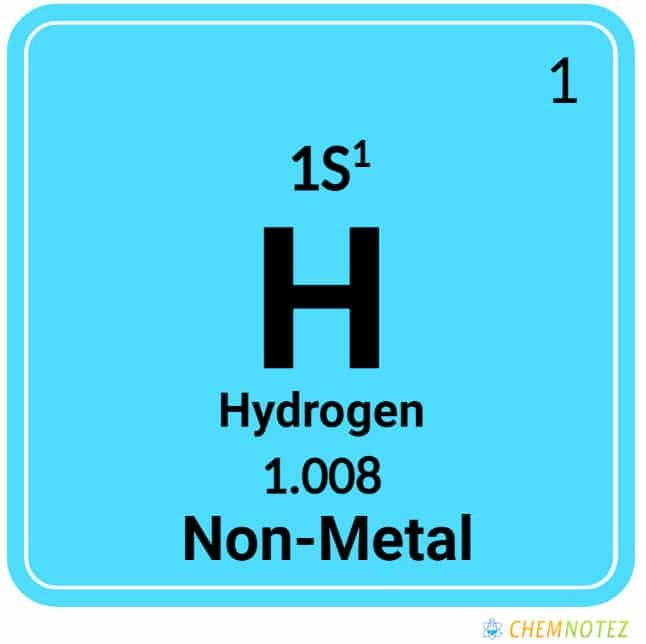
| Atomic mass | 1.008 amu |
| Weight | 1.00794 |
| Density | 0.08988 g/L |
| Atomic Radius | 53 pm |
| Covalent Radius | 31 pm |
| Ionic Radius | 120 pm |
| Ionization Energy | 13.6eV |
| Electronic Gain Enthalapy | -73 |
| Electron Negativity | 2.2 |
| Electron Affinity | 72.8 kJ/mol |
| Melting Point | 13.99k (-259.16 °C, -434.49 °F) |
| Boiling Point | 20.271 K (−252.879 °C, −423.182 °F) |
Chemical Properties of Hydrogen
| Atomic Number | 1 |
| Group | 1 |
| Period | 1 |
| Block | S |
| Electronic configuration | 1s1 |
| Combustion | Combustible |
| Chemical Reactivity | Highly Reactive |
| Valancey of Element | 1 |

Lewis Dot Structure of Hydrogen
The Lewis dot structure of hydrogen is H⋅H⋅ because it has one valence electron.

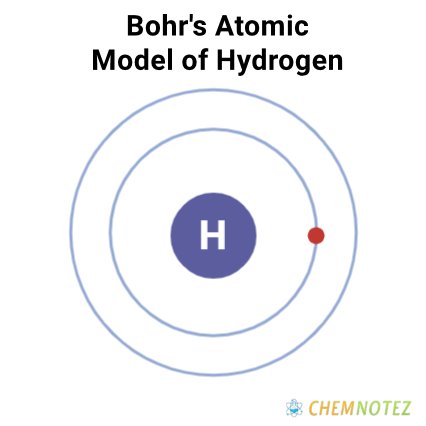
Bohr’s Atomic Model of Hydrogen
Different States of Hydrogen
Molecular form (Gaseous Phase)
- Hydrogen is gas in molecular form with a dual-atomic structure (H2).
- It consists of two protons and two electrons.
- Hydrogen is unique in the sense that it does not have a neutron, but it’s the very same reason why it’s able to bond to many elements.

Metallic form
- Hydrogen in metallic form is pressurized until it becomes liquid with particular electrical conductive properties.
- It is called metallic not because electrons move easily around it.
- Metallic property of hydrogen is inspected through the use of diamonds and lasers.
Dark form
- In the dark form, hydrogen is not in its gaseous or metallic state, but instead acts up in the middle of the two.
- Hydrogen in dark form doesn’t send out light.
- It does not have the ability to reflect light.
- What it does is it sheds thermal energy.
What are the common uses of Hydrogen
Hydrogen is one of the chemical elements with plenty of uses. The most common ones include the refining of metals, making ammonia for fertilizers, and methanol for different types of plastic materials. Let us dig deeper into the different uses of hydrogen.
Industrial processes
- Hydrogen is useful in industrial processes such as treating metals and refining petroleum, especially in the United States where hydrogen is used to minimize the sulfur content of fuels.
- Hydrogen is also used in the food processing industry.
Exploring outer space
- Hydrogen plays an important role in outer space exploration.
- It is used as rocket fuel, where hydrogen is mixed with liquid oxygen to create a powerful explosion.
- The National Aeronautics and Space Administration (NASA) started using hydrogen in liquid form as early as the 1950s.
- It is also the first to use hydrogen fuel cells in powering the spacecraft’s electrical system.
Electricity
- Hydrogen is also used to produce electricity.
- The hydrogen fuel cells do this by combining the atoms of hydrogen and oxygen.
- The combination of the two atoms creates a reaction that is similar to that of the battery, thereby producing electricity, water, and heat.
- Hydrogen fuel cells are used in a wide array of applications, such as powering cellphones and laptop computers.
- Large fuel cells can power electrical power grids.
- They can also power places that are not connected to electric power grids, as well as act as a power backup in case of a power outage.
Hydrogen used to power vehicles
- Under the Energy Policy Act of 1992, hydrogen can be used as an alternative fuel for vehicles.
- Hydrogen can power fuel cells in zero-emission vehicles.
- The fuel cells can be two to three times more efficient than the internal combustion engine that runs on gasoline.
- Hydrogen also has the ability to fuel internal combustion engines.
- There are car manufacturers that have light-duty hydrogen fuel cell cars and most of them are available in California, where there are a multitude of hydrogen fueling stations.
Electricity generation
- The combustion of hydrogen is useful in the generation of electricity.
- There is a growing interest in the use of hydrogen as a power plant fuel.
- In America alone, there are a number of power plants that plan to operate using natural gas-hydrogen fuel mixtures in combustion gas turbines.
Price of Hydrogen
The cost of hydrogen fluctuates depending on market demand and type. The cost of green hydrogen ranges between $2.50 and $6.80 per kilogram. High-carbon grey hydrogen costs around $1.80 per kilogram. Blue hydrogen ranges between $1.40 and $2.40 per kilogram, while turquoise hydrogen ranges between $1.40 and $2.40 per kilogram.
Interesting facts about hydrogen
- Don’t you know that, according to scientists, hydrogen makes up over 90% of all the atoms in the universe.
- Hydrogen is the only chemical element that does not have neutrons.
- You can produce hydrogen in a laboratory by combining acid (diluted form) with any metal.
- Hydrogen is extremely light, so it was used in lighter-than-air balloons. The main concern is its highly flammable nature, which makes it extremely dangerous.
- Around 10% of the human body mass is hydrogen.
- Hydrogen, although gas, becomes liquid when exposed to an extremely low temperature and high pressure.
- Hydrogen becomes liquid metal under very high pressure.
- It is believed that metallic hydrogen is present at the core of Jupiter, which is a gas giant planet.
Frequently Asked Questions
Q1. Why is hydrogen referred to as an energy carrier?
Hydrogen is not an energy source. However, it can carry the energy produced from other sources, so it is called an energy carrier.
Q2. Is there any danger associated with handling hydrogen?
One of the primary concerns with regard to handling hydrogen is safety. Hydrogen is highly flammable. It has an almost invisible flame that, if not handled properly, could lead to accidental burns.
Q3. Why do we need hydrogen?
The body needs hydrogen because it is a significant water element and because it enables the body to produce energy.
Q4. Is hydrogen not always clean?
Hydrogen is not always clean. Its cleanliness primarily depends on its inputs. Fortunately, there are now technologies that produce clean hydrogen and it usually involves the process of electrolysis (electricity to water).
Q5. Is hydrogen refueling station safe?
Hydrogen refueling stations are safe. It is built in accordance with the safety standards set by the governing bodies. Currently, there are 300 hydrogen refueling stations in the world.
References
- https://www.lenntech.com/periodic/elements/
- https://www.rsc.org/periodic-table/element/1/hydrogen
- https://www.britannica.com/science/hydrogen
- https://en.wikipedia.org/wiki/Hydrogen
- https://pubchem.ncbi.nlm.nih.gov/element/Hydrogen
- https://www.livescience.com/28466-hydrogen.html
- https://www.chemicool.com/elements/hydrogen.html
- https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/hydrogen.php
- https://www.thoughtco.com/hydrogen-element-facts-606474
- https://byjus.com/chemistry/hydrogen/