What is Indium?
Soft, pliable metal with a silvery sheen – that is Indium. It is always found in trace amounts of other minerals such as lead and zinc. Indium is usually extracted as a byproduct of these elements. Indium is unique in the sense that it can be easily scratched with a fingernail and can be twisted and formed into any shape.
Where is Indium obtained?
Seldom does indium occur alone in nature? It is generally found in ores of zinc, copper, iron, lead, and copper. It is more abundant than silver or mercury. In the Earth’s crust, it is thought to make up about 0.1 parts per million (ppm). Around 75 tons of commercial indium are imported annually from Canada. More than 1,500 tons of the metal are in reserves. When compared to non-cultivated soils, cultivated soils are occasionally found to contain higher concentrations of indium.
History of Indium
Ferdinand Reich, a German chemist made the discovery of indium in 1863 at the Freiberg School of Mines in Germany. In order to determine whether a sample of a zinc mineral mixture might include the freshly discovered element thallium, Reich was examining it. He first roasted the ore to burn out the majority of the sulfur, then he treated the residual components with hydrochloric acid.

He noticed a solid yellowish material form. Because he was color blind, he invited colleague, Hieronymous T. Richter to look at the sample’s spectrum because he thought it might be the sulfide of a brand-new element.Richter noticed a vivid violet line that did not correspond to any known element’s spectral line.
Together, the two researchers were able to isolate the new element’s sample and make the announcement of its discovery. In honor of the Latin word indicum, which means violet, they gave the new element the name indium.
Classification, Properties and Characteristics of Indium
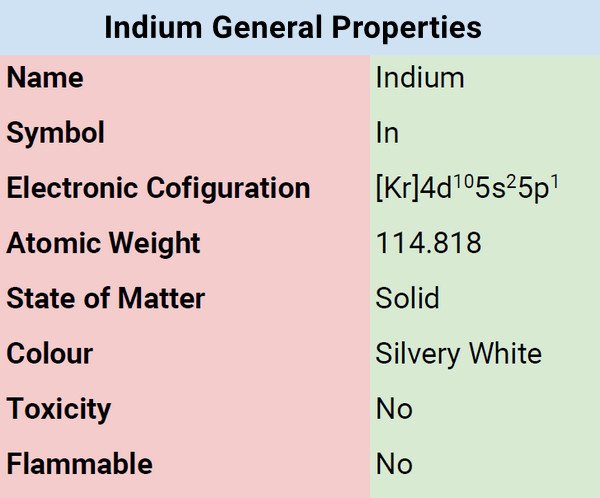
Indium is an extremely malleable, silvery-white, and glossy metal. Glass and other comparable surfaces either stick to or get wet from indium liquid.
Indium is solid at room temperature, but like gallium, maintains its liquid state across a broad temperature range. Indium is primarily found in the oxidation state III when it is present in compounds. It emits a violet flame when heated over its melting point.
Lewis Dot Structure of Indium

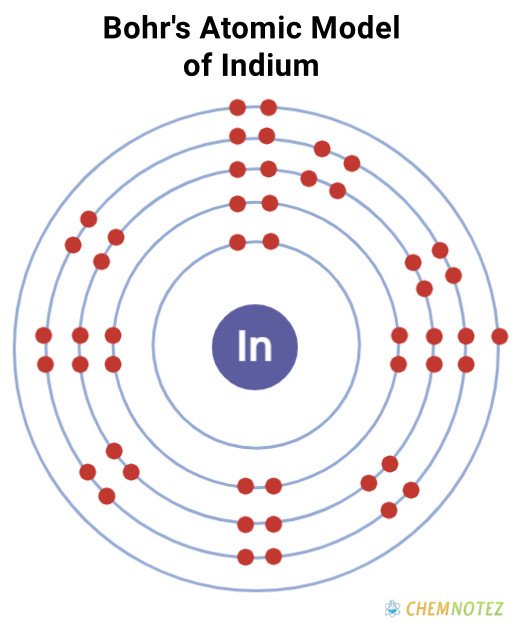
Bohr’s Atomic Model of Indium
Atomic Data of Indium
Physical Properties of Indium
| Color | Silvery |
| Odor | Odorless |
| Taste | Tasteless |
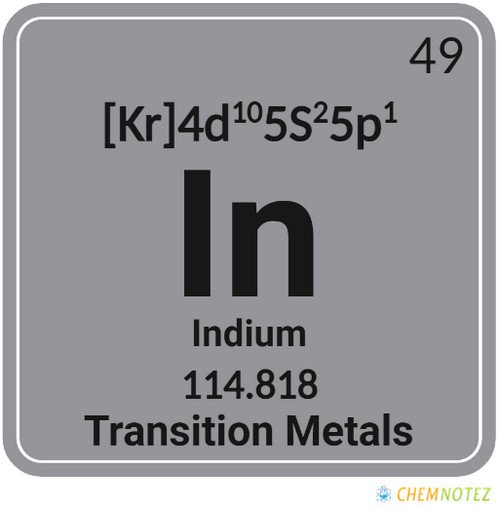
| Atomic Mass | 114.818 |
| Weight | 114.82 g.mol -1 |
| Density | 7.31 |
| Atomic Radius | 1.93 Å |
| Ionization Energy | 558.299kJ mol−1 |
| Covalent Radius | 1.42Å |
| Ionic Radius | 0.092 nm (+2) |
| Electronic Gain Enthalpy | 28.9 kJ mol−1 |
| Electron Negativity | 1.78 |
| Electron Affinity | 28.9 kJ mol−1 |
| Melting Point | 156.60°C, 313.88°F, 429.75 K |
| Boiling Point | 2027°C, 3681°F, 2300 K |
Chemical Properties of Indium
| Atomic Number | 49 |
| Group | 13 |
| Period | 5 |
| Block | p |
| Electronic Configuration | [Kr] 4d105s25p1 |
| Combustion | Flammable in dust and powder form |
| Chemical Reactivity | Reactive |
| Valency of Element | 3 |
Different States of Indium
Indium is a post-transition metal, solid at room temperature. However, it melts once desired temperature is reached. In fact, it has a low melting point. It can be bent into your desired shapes once the desired temperature is reached, which is typically around 107 degrees to 315 degrees Fahrenheit.

Uses of Indium
- Indium compounds, specifically indium tin oxide, are essential to the global economy (ITO). For the increased demand for LCDs (liquid crystal displays) in touch screens, flat-screen TVs, and solar panels, ITO continues to be the ideal material. ITO is transparent, transmits electricity, sticks firmly to glass, and is resistant to corrosion. It is robust mechanically and chemically which makes it ideal for LCDs and other flat-panel displays.
- ITO is frequently used to coat mirrors and glass. When applied to windshields of vehicles or planes, it can greatly lessen the need for air conditioning and enables the glass to de-ice or de-mist.
- Indium is tagged as the “metal vitamin,” because it is frequently utilized to create alloys and is known to make a significant difference in an alloy even at very low concentrations.
- High-speed motor bearings and other metal surfaces are covered with indium alloys.
- Indium metal is ideal for use in tools and equipment required in extremely cold environments because it retains an uncommon amount of softness and malleability at very low temperatures. Examples are high vacuum systems and cryogenic pumps.
- Sprinkler heads, fire-door linkages, and fusible plugs are made from its low-melting indium alloys.
- Indium’s stickiness makes it ideal to use as a solder.
- The metal indium is used to create a variety of electrical components, including photoconductors, thermistors, and rectifiers, which change alternating electricity into direct current.
- Indium nitride is a semiconductor commonly used in microchips and transistors.
- Indium is applied to glass materials to give them a mirror finish, which is common in tall buildings.
- Indium is used as a protective film on the goggles used by welders.
- Indium’s low friction property is the main reason why it is used to coat formula 1 racing cars’ ball bearings.
- Indium alloy’s low melting point is the reason why it is used in fire sprinkler systems.
Price of Indium
The cost of indium fluctuates depending on supply and demand. It also depends on quantity and purity. Pure indium costs around $1 to $5 per gram.
Interesting facts about Indium
- Indium ranked 61st in the list of the most common elements in the earth’s crust.
- There are a total of less than 10 indium minerals and none of them is present in the earth’s major deposits.
- Indium is not reactive with water.
- Indium’s boiling and melting points are lower than metals belonging to the transition metal group.
- Don’t you know that if you bend indium, it is said to scream? It is quite the same as cry in tin.
Frequently Asked Questions
Q1. Is indium toxic to humans?
There is growing proof that indium can be extremely harmful, particularly in work environments where indium lung disease—a potentially fatal condition brought on by inhaling indium particles is known to occur.
Q2. What does indium react with?
At ambient temperature, oxygen does not react with indium metal, but it does dissolve in acids. Indium oxide is created when it reacts with oxygen at higher temperatures.
Q3. Can indium be reused?
Yes. In fact, about 86% of indium can be reused and recycled.
Q4. How much indium is left in the world?
Indium is abundant in the crust of the earth, according to the United States Geological Survey (USGS). The estimated number of indium in the earth’s crust is 240 parts per billion in terms of weight. Thus, making indium three times more than silver.
Q5. What can replace indium?
Indium is expensive, which is one of the hindrances for some industries to use it. Metals that can substitute for indium are tin, zinc, and gallium. Such metals are lower in cost and readily available.
References
- https://www.livescience.com/37352-indium.html
- https://www.rsc.org/periodic-table/element/49/indium
- https://www.britannica.com/science/indium
- https://www.lenntech.com/periodic/elements/in.htm
- https://www.chemicool.com/elements/indium.html
- https://www.thoughtco.com/indium-facts-606545
- https://en.wikipedia.org/wiki/Indium
- https://study.com/academy/lesson/indium-element-facts-discovery-isotopes.html
- https://www.acs.org/content/acs/en/greenchemistry/research-innovation/endangered-elements/indium.html
- https://www.chemistrylearner.com/indium.html