What is Iodine?
Iodine is a crucial component of life. It is known primarily for the crucial part it plays in the generation of thyroid hormones in both humans and other animals. You will be able to know more about this interesting element in this article.
Where is Iodine obtained?
In nature, iodine is never found alone and is never concentrated enough to form standalone minerals. It only occurs in trace amounts in seawater, at a rate of around 50 mg per metric ton. Additionally, it develops in cod livers, oysters, and seaweed. Crude Chilean saltpetre contains sodium iodate. Thyroxine, a substance made by the thyroid gland and found in the human body, contains iodine.
History of Iodine
When making saltpetre in 1811, Bernard Courtois, a French chemist, heated seaweed ashes using sulfuric acid to produce a violet vapor as a byproduct. In 1813, British chemist Sir Humphry Davy, who was traveling through Paris on his way to Italy, identified this vapor as an element similar to chlorine and proposed the name iodine. It is derived from the Greek word ioeides, which means “violet-colored.” This vapor then condensed to a black crystalline substance, and referred to as a “substance X.”

Classification, Properties and Characteristics of Iodine
Iodine is a solid non-metal element classified as halogen, which means that it is one of the salt-producing elements. When it combines with metals, it forms a wide array of salts such as sodium chloride, calcium fluoride, potassium iodide, and silver bromide.
It is characterized by its glossy purple-black color in its pure form. It easily sublimes and emits a purple vapor as it transitions from a solid to a gaseous state. Despite theoretically being a non-metal, it possesses some metallic characteristics.

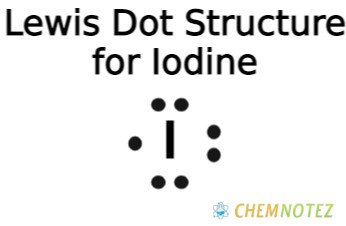
Lewis Dot Structure of Iodine
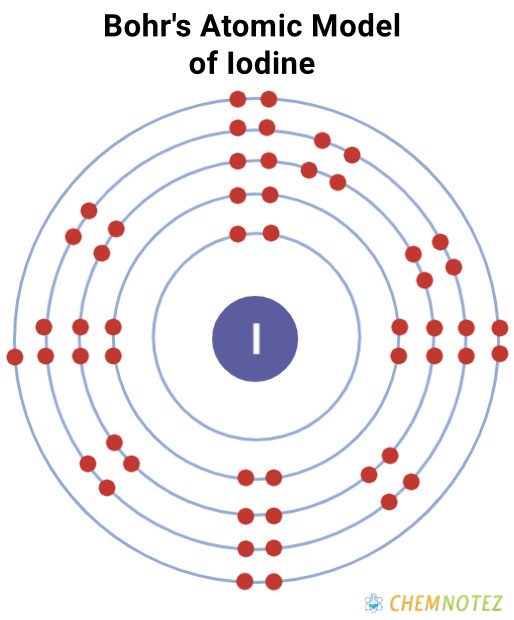
Bohr’s Atomic Model of Iodine
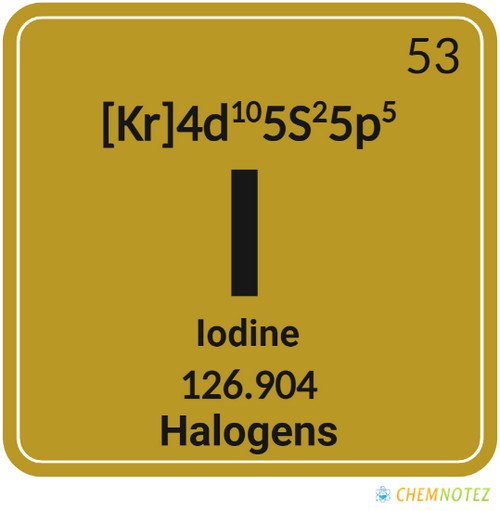
Atomic Data of Iodine
Physical Properties of Iodine
| Color | Blue-black with metallic sheen |
| Odor | Strong, harsh odor |
| Taste | Metallic taste |
| Atomic Mass | 126.904 |
| Weight | 126.9044 |
| Density | 4.933 |
| Atomic Radius | 1.98 Å |
| Ionization Energy | 1008.393kJ mol−1 |
| Covalent Radius | 1.36Å |
| Ionic Radius | 0.216 nm (-1) ; 0.05 nm (+7) |
| Electronic Gain Enthalpy | 295.152 kJ mol−1 |
| Electron Negativity | 2.66 |
| Electron Affinity | 295.152 kJ mol−1 |
| Melting Point | 113.5 °C (236 °F) |
| Boiling Point | 184 °C (363 °F) |
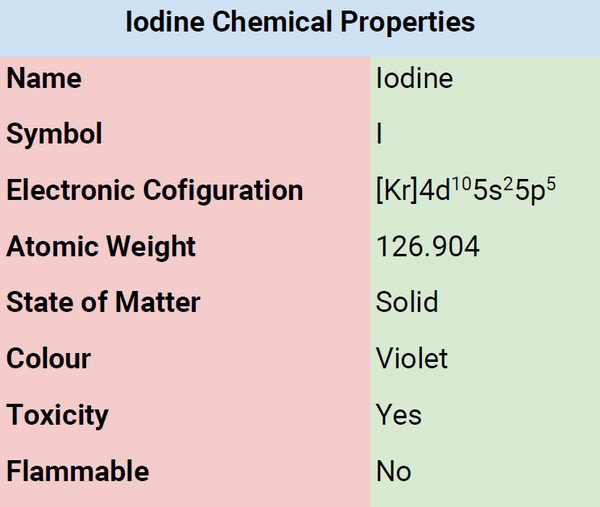
Chemical Properties of Iodine
| Atomic Number | 53 |
| Group | 17 |
| Period | 5 |
| Block | p |
| Electronic Configuration | [Kr] 4d105s25p5 |
| Combustion | Not Combustible |
| Chemical Reactivity | Least Reactive |
| Valency of Element | -1 |
Different States of Iodine
At standard conditions, iodine is solid. It melts once desired temperature is reached, and when it melts, it forms a deep violet liquid. When you bring it to a boil, it emits a violet-colored gas.
Uses of Iodine
- The first commercial application of iodine is in photography. In 1839, Louis Daguerre came up with a method to produce images on a piece of metal which was called daguerreotypes.
- Iodide salts are widely used in the pharmaceutical industry.
- It is used in dyes and printing inks.
- It is used as a disinfectant.
- It is used as an animal feed supplement.
- It is used to polarize filters for LCD displays.
- Table salts are added with iodide to avoid iron deficiency, which could significantly affect the thyroid gland.
- Iodine’s radioactive isotope, iodine-131 can be used to treat cancerous thyroid glands.
- It is one of the elements essential to humans. The body needs around 0.1 mg of iodide. It is beneficial to the thyroid gland; a gland that promotes growth and body temperature. A deficiency in iodine causes the thyroid gland to swell up leading to a condition called goiter.
- Iodine compounds are used to disinfect external wounds such as iodine in alcohol and a solution containing potassium iodide.
- Lugo’s iodine solution is widely used in a laboratory setting. it is an aqueous solution characterized by its purple color that helps scientists detect the existence of starch in a substance.
Price of Iodine
The cost of iodine fluctuates depending on the supply and demand. Iodine in pure form costs around $8 per 100 grams. The cost is cheaper if you purchase in bulk.
Interesting facts about Iodine
- The origin of the word iodine is iodes, a Greek word that means violet. It is called that way because iodine vapor is colored violet.
- iodine plays a crucial role in the drug industry. Since it is a halogen, it can be used to improve the drug’s properties.
- Don’t you know that there is more iodine in the ocean than in the earth’s crust?
- Seaweed is one of the food sources that have the highest iodine concentration.
- Iodine-127 is the only naturally-occurring iodine isotope. It exists in nature on its own.
- Iodine-129 has the ability to change from radioactive to non-radioactive form over the span of millions of years.
- The majority of the body’s daily iodine need is derived through diet by eating foods rich in iodine such as seaweed.
- Don’t you know that iodine is the heaviest element vital for human health and life?
- Iodine is primarily derived from food sources. Foods that are high in iodine are seaweed, fish, iodized salt, some fruits and vegetables, and dairy products like yogurt, cheese, and milk. Marine life is rich in iodine such as shrimps and salmons.
- The need for iodine is high in pregnant women. That is why pregnant women are usually given iodine supplements.
- The intake of iodine must be regulated. Too much iodine in the body can be detrimental to one’s health.
Frequently Asked Questions
Q1. Which fruit is rich in iodine?
Although marine life is the main source of iodine, it’s surprising to know that some fruits have a high level of iodine. Examples are pineapple, cranberries, and strawberries.
Q2. What is the effect of iodine on the thyroid gland?
The thyroid gland heavily relies on iodine and transforms it into thyroid hormones, specifically triiodothyronine and thyroxine. The thyroid cells consume iodine in the body. The thyroid cells bind together to form the amino acid tyrosine and T3 and T4.
Q3. Does iodine have the ability to detoxify the body?
Yes. Iodine in the right amount can help get rid of toxins from the body. Examples of these toxins are ammonia, bromine, and pesticides.
Q4. How long will it take for iodine to work?
It usually takes around two to three months to noticeably see the results of iodine, especially if you are taking it for thyroid gland purposes. A single dose of iodine is helpful in treating hyperthyroidism but usually takes three to six months for the results to kick in.
Q5. Can cooking destroy iodine?
Some food preparation methods and techniques can cause iodine loss, which is about 3% to 67% depending on the cooking methods.
References
- https://www.livescience.com/37441-iodine.html
- https://www.britannica.com/science/iodine/Physical-and-chemical-properties
- https://www.rsc.org/periodic-table/element/53/iodine
- https://www.lenntech.com/periodic/elements/i.htm
- https://www.thoughtco.com/facts-about-iodine-607974
- https://www.chemicool.com/elements/iodine.html
- https://chemistrytalk.org/iodine-element/
- https://www.ducksters.com/science/chemistry/iodine.php
- https://byjus.com/chemistry/iodine/
- https://www.factsjustforkids.com/chemistry-facts/iodine-facts-for-kids/