What is Neon?
Neon is an inert gas with the symbol “Ne” and an atomic number of “10.” It is colorless and odorless and has an equivalent of 10 protons and electrons. It belongs to the noble gas group because of its highly unreactive nature.

Where is Neon obtained?
Neon is primarily obtained from liquefying air. It does not come from mines, but from the air. The air needs to undergo the process of distillation of cryogenic liquified air. It is the fifth most abundant element in the universe.
History of Neon
The neon element was discovered in 1898 by British chemists Morris Travers and William Ramsay, Ramsay boiled a liquid air sample and, as the gases evaporated, he captured it through the process of fractional distillation.
He identified and ruled out oxygen, nitrogen, argon, and krypton. He eventually discovered a red-orange light, which turned out to be neon. He named it after the Greek word “neos”, which means “new.”
Classification, Properties and Characteristics of Neon
Neon is a chemical element that is light and belongs to the inert gas family. It is colorless under normal conditions. When placed in a vacuum discharge tube, it glows in a reddish-orange color. It does not form any known stable compounds.

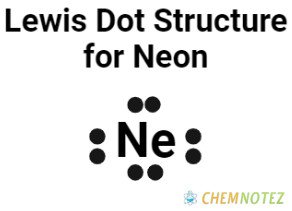
Lewis Dot Structure of Neon
Bohr’s Atomic Model of Neon

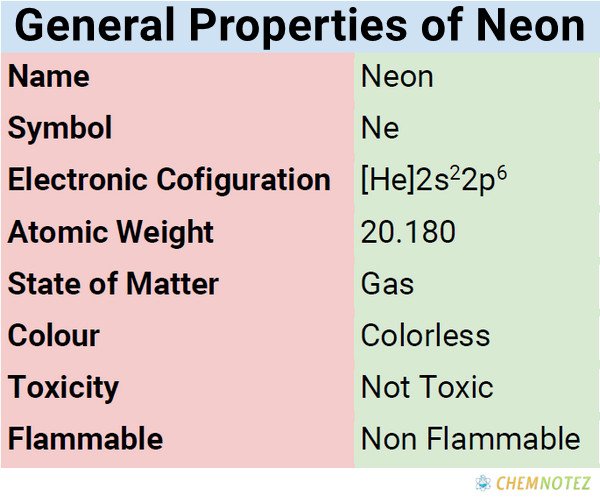
Atomic Data of neon
Physical Properties of Neon
| Color | colorless/ orange-red |
| Odor | odorless |
| Taste | tasteless |
| Atomic Mass | 20.180 |
| Weight | 20.1797 |
| Density | 0.000825 |
| Atomic Radius | 154 pm |
| Ionization Energy | 21.5645 eV |
| Covalent Radius | 58 pm |
| Ionic Radius | 154 pm |
| Electronic Gain Enthalpy | +116kj mol-1 |
| Electron Negativity | unknown |
| Electron Affinity | -1.2(2) eV |
| Melting Point | -248.59°C, −415.46°F, 24.56 K |
| Boiling Point | −246.046°C, −410.883°F, 27.104 K |
Chemical Properties of Neon
| Atomic Number | 10 |
| Group | 18 |
| Period | 2 |
| Block | p |
| Electronic Configuration | [He]2s²2p⁶ |
| Combustion | It is a non-flammable gas |
| Chemical Reactivity | it does not react with other substances |
| Valency of Element | 0 |
Different States of Neon
When neon is placed under low pressure, it emits a bright orange-red light, especially when the electrical current is passed through it. It liquefies at -411°F and freezes at 21/2° lower.

What are the common uses of neon?
Advertising
- Neon is widely used for advertising purposes. When volts are applied to neon, it emits light that is orange to bright red in color. It was Georges Claude who first created glass tubes of neon in 1910.
- He bent the glass tubes to create glowing letters. It was eventually used for advertising signs because they glowed and would attract the attention of people.
Cryogenic refrigerant
- Neon in liquid form is used as a cryogenic refrigerant. Neon has 40 times more refrigerating capacity per unit volume when compared to liquid helium.
Warning indicators
- Neon is very good at giving warning indicators, especially in high-voltage areas, lightning arresters, switching gears, lasers, and diving equipment.
Creation of helium-neon lasers
- Neon and helium atoms get excited when in contact with electric currents.
- The atoms of these two chemical elements collide and transfer and release energy, which results in amplifying the light inside the tube and eventually producing a laser.
- The resulting laser emits bright red light with an equivalent wavelength of 632.8 nm.
Price of neon
The current market price of pure neon is $33 per 100 grams. You can save money if you buy in bulk.
Interesting facts about neon
- Don’t you know that helium is the second lightest chemical element that belongs to the noble gas family?
- Don’t you know that neon has the narrowest liquid range of all the chemical element? It is only 2.5 degrees Celsius.
- Although neon is rare on Earth, it ranks fourth on the list of the most abundant elements in the universe.
- Neon is widely known in the world for its usability in signage and advertising, specifically neon signage characterized by orange to reddish light.
- About two-thirds of the air’s density is neon. For the reason that helium is lighter than neon, a balloon consists of neon floats but rises slower when compared to balloons with helium.
- If you breath in neon, your voice will have a distinct high-pitched sound. Its effect is almost the same as that of helium.
- Don’t you know that neon in liquid form can be used to free corpses?
- Neon is one of the few elements that does not form compounds when it gets in contact with other elements. It does not have a stable compound.
- A room filled with neon is equivalent to two gallons of neon gas.
- Don’t you know that neon is one of the rarest chemical elements on earth?
- Don’t you know that if you put neon in a tube, it will emit a light characterized by a reddish-orange color?
- Don’t you know that the stars have neon? It is present during the alpha process of stars as a result of the fusion of oxygen and helium.
- Don’t you know that the average home in the United States has about 10 liters of neon?
- Don’t you know that a neon light has the ability to penetrate fog?
- Neon is one of the most expensive chemical elements.
Frequently Asked Questions
Q1. What happens if you breathe in neon?
Neon is an inert gas and belongs to the simple asphyxiant family. If you inhale too much neon, it could lead to a variety of clinical symptoms like nausea, vomiting, dizziness, loss of consciousness, and even death. A person who inhales a high concentration of neon must receive first aid right away as it can be extremely fatal.
Q2. How can neon inhalation lead to death?
As a simple asphyxiant, neon can get rid of oxygen from the air, especially in a closed space. If you are contained in a room with a high level of neon, the oxygen from the air is gradually removed, and you will eventually find it hard to breathe until such time that you will die from suffocation.
Q3. What gives neon its distinct color?
Neon has an orange-reddish color and the one responsible for it is the electrode of a particular noble gas as they release a particular wavelength of photons. If you put it inside a glass tube, the color will change to classic red.
Q4. How long does a neon light last?
A neon light can last between eight and 15 years. Some may last far longer than 15 years.
Q5. Can neon burn?
Neon does not burn. In fact, it will not burn you when you touch it. It can be warm but not that warm to cause burning. However, the electrodes attached to neon tubing get hot and should not be touched.
References
- https://www.rsc.org/periodic-table/element/10/neon
- https://www.britannica.com/science/neon-chemical-element
- https://www.thoughtco.com/interesting-neon-element-facts-4077247
- https://www.livescience.com/28811-neon.html
- https://www.chemicool.com/elements/neon.html
- https://chemistrytalk.org/neon-element/
- https://www.lenntech.com/periodic/elements/ne.htm
- https://en.wikipedia.org/wiki/Neon
- https://www.mentalfloss.com/article/567681/element-neon-facts
- https://pubchem.ncbi.nlm.nih.gov/element/Neon