What is Sodium?
Sodium is known to be a partner of chlorine to form sodium chloride or popularly known as table salt, but don’t you know that sodium alone can be explosive? It is highly reactive when in contact with other elements.

Aside from being a primary component of table salt, sodium is also used in sodium peroxide, baking soda, and sodium borate or borax. Sodium also plays a crucial role in controlling blood pressure and maintaining the function of the nervous system.
Where is Sodium obtained?
Sodium is present in the stars and sun. In fact, it’s the fourth most abundant chemical element on earth. Commercially, sodium is obtained through the process of electrolysis involving fused sodium chloride. It is commonly used because it is cheaper when compared to sodium hydroxide electrolysis.

History of Sodium
Sodium is derived from the Latin word “sodanum” and the English word “soda”, and was discovered by the English chemist Humphry Davy in 1807 from caustic soda electrolysis. He did this to attain pure sodium.
In prehistoric times, sodium was used for many reasons, such as manufacturing glasses, relieving headaches, and flavoring. Various sodium compounds are from various sources. Sodium chloride, for example, was derived from seawater, and sodium carbonate from the ashes of rare plants.
Classification, Properties and Characteristics of Sodium
One known characteristic of sodium is its hyperactivity. It reacts to almost all non-metals. Sodium is stored in an inert liquid to avoid reactions. If sodium is exposed to air, it reacts with oxygen, forming sodium oxide. It will then react with hydrogen to form sodium hydroxide. If sodium is burned in the air, it leads to sodium peroxide. That’s how chemically reactive sodium is.

Lewis Dot Structure of Sodium

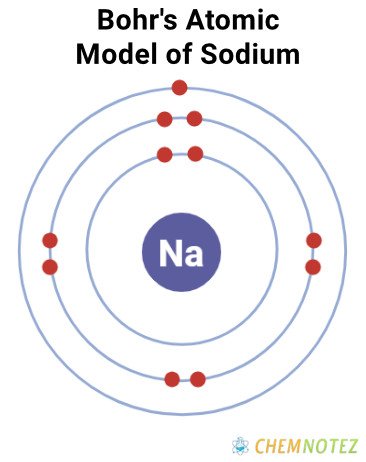
Bohr’s Atomic Model
Atomic Data of sodium
Physical Properties of Sodium
| Color | soft silvery white |
| Odor | odorless |
| Taste | salty |
| Atomic Mass | 22.990 |
| Weight | 22.989769 u |
| Density | 0.97 |
| Atomic Radius | 2.27 Å |
| Ionization Energy | 5.139 eV |
| Covalent Radius | 1.66 Å |
| Ionic Radius | 227 pm |
| Electronic Gain Enthalpy | 5.1 eV. |
| Electron Negativity | 0.9 |
| Electron Affinity | 52.867 |
| Melting Point | 97.794°C, 208.029°F, 370.944 K |
| Boiling Point | 882.940°C, 1621.292°F, 1156.090 K |
Chemical Properties of Sodium
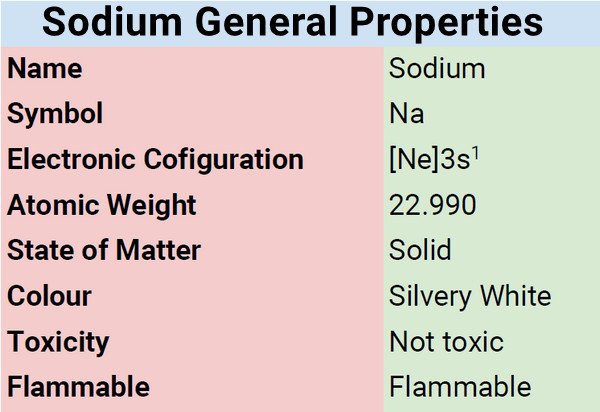
| Atomic Number | 11 |
| Group | 1 |
| Period | 3 |
| Block | s |
| Electronic Configuration | [Ne] 3s1 |
| Combustion | It is a combustible solid that ignites in air |
| Chemical Reactivity | It is reactive to halogens but not with carbon |
| Valency of Element | 1 |
Different states of Sodium
At room temperature, sodium is solid. However, it melts at the right temperature, specifically 97.80 degrees Celsius. Sodium in elemental form is a liquid metal such as solder and mercury. It has the ability to transfer heat and can keep the core cool even without external power.

What are the common uses of Sodium?
Manufacturing industry
- Metallic sodium is useful in the manufacturing industry, specifically in manufacturing esters and sodamide.
Alloy modification
- Metallic sodium is used in modifying alloys to improve their fluidity and mechanical properties. It also smooths the surface of metals and purifies molten metals purification.
Heat transfer agent
- Sodium is an effective heat transfer agent. It can cool nuclear reactors.
Nutrition
- Sodium chloride plays a crucial role in maintaining a healthy body. Sodium ions are useful in transmitting electrical signals in the nervous system.
- It regulates the water balance between body fluids and cells in the body.
Forming various compounds for various uses
- Sodium plays a vital role in forming compounds to be used for various industries, such as sodium peroxide, sodamide, sodium hydride, sodium chloride, baking soda, chile saltpeter, borax, and sodium cyanide.
Light production
- Sodium vapor is used in creating lights such as brilliant yellow lights and streetlights.
Glass making
- Solid sodium carbonate is necessary for making clear glass. Sodium carbonate is heated with calcium oxide.
Sodium as a catalyst
- Sodium serves as a catalyst in various reactions, such as in the making of artificial rubber.
Conducts various bodily processes
- A small amount of sodium is needed by the body to conduct nerve impulses.
- It also plays an important role in muscle contraction and relaxation. To achieve these vital functions, the body needs at least 500 mg of sodium on a daily basis.
Soap making
- Sodium is one of the key ingredients in making soap. It is usually combined with fatty acids.
Price of Sodium
Sodium is more affordable when compared to other chemical elements. A gram of sodium costs around $0.50 – $1 USD.
Interesting facts about Sodium
- Sodium is less dense than water. In fact, the metal form of sodium easily floats on top of water.
- Sodium at room temperature is so soft that it can be cut using a butter knife. It is also soft and shiny.
- Sodium metal is highly reactive to water. Once it gets into contact with water, it forms hydrogen gas and can ignite and create the appearance of sodium burning.
- If you burn sodium, it creates a bright yellow light, which is why sodium is one of the components used in making fireworks.
- Sodium is one of the most abundant elements in the human body. About 0.15% of the body is sodium.
- Don’t you know that sodium is ranked sixth in the list of most abundant elements on earth?
- Don’t you know that ionizing sodium vapor can lead to the creation of yellow light, which is commonly found in street lights?
- Don’t you know that sodium chloride is the most common sodium compound? It is commonly known as table salt.
- Sodium is an alkali metal, and one of the characteristics of alkali metals is that their density increases as their atomic number increases. However, sodium has a higher density when compared to potassium.
Frequently Asked Questions
Q1. Why does sodium need to be stored under kerosene?
Because of the highly reactive nature of sodium, it needs to be properly stored, or say, specially stored. It does react with oxygen and moisture easily, which is most likely to happen when stored in an open space. To prevent any reaction, it should be immersed in liquid hydrocarbons such as kerosene.
Q2. What makes sodium highly reactive?
Sodium belongs to the alkali metal group, also known as group 1 in the periodic table. It has one electron in the outermost shell, which is the very reason it has a low ionization energy. It is highly reactive for the very reason that its electrons can be removed easily.
Q3. Why is sodium soft?
Although sodium belongs to the alkali metal group, it is soft primarily because of its weak metallic bonding.
Q4. What is the function of sodium in the body?
Sodium is one of the elements that plays an important role in the body. It does play a crucial part in muscle relaxation and contraction. It also facilitates the transmission of neural inputs. A healthy level of sodium must be maintained because any excess can increase the body’s blood pressure.
Q5. What foods are rich in sodium?
The body needs sodium for various bodily processes, but too much sodium is bad. The maximum daily sodium intake must be 500 mg. For foods high in sodium, the list includes pizza, salted nuts, burritos, cured meat, and fish, including canned and salted goods.
References
- https://www.lenntech.com/periodic/elements/na.htm
- https://www.rsc.org/periodic-table/element/11/sodium
- https://www.britannica.com/science/sodium
- https://www.livescience.com/28820-sodium.html
- https://www.thoughtco.com/sodium-element-facts-606471
- https://www.chemicool.com/elements/sodium.html
- https://pubchem.ncbi.nlm.nih.gov/element/Sodium
- https://en.wikipedia.org/wiki/Sodium
- https://byjus.com/chemistry/sodium/
- https://chemistrytalk.org/sodium-element/