What is Tellurium?
Tellurium is a silver-white, semi-metallic, glossy, crystalline, and brittle element. It is often sold as a dark gray powder. As a semi-metallic element, it possesses both metallic and nonmetallic characteristics. Numerous tellurium compounds resemble those of sulfur and selenium. Tellurium emits a greenish-blue flame when burned in the air. Water and hydrochloric acid do not affect tellurium. Although it can be dissolved by nitric acid.
Where is Tellurium obtained?
There are only 0.001 parts per million of tellurium in the crust of the Earth. Some of the minerals that contain tellurium are calaverite (gold telluride), Sylvanite, and tellurite. Today, the majority of tellurium is sourced from byproducts of copper mining and refining. It is derived commercially from the anode muds.

History of Tellurium
Franz Joseph Müller von Reichenstein was the person behind the discovery of tellurium in 1783 in Sibiu, Romania. A metallic-looking ore from a mine near Zalatna that piqued his interest was thought to be either local antimony or bismuth. The preliminary investigation revealed that neither antimony nor bismuth was present (it was actually gold telluride, AuTe2).

Müller investigated the ore for three years before establishing that it included a novel ingredient. His research was published in an obscure journal, where it received little attention. Martin Klaproth in Berlin, to whom he sent a sample in 1796, verified his findings.
Klaproth created a pure sample and chose the name “tellurium” for it. Strangely enough, this wasn’t the first time he had handled tellurium. Paul Kitaibel, a Hungarian scientist who had discovered it independently, had sent him some in 1789.
Classification, Properties, and Characteristics of Tellurium
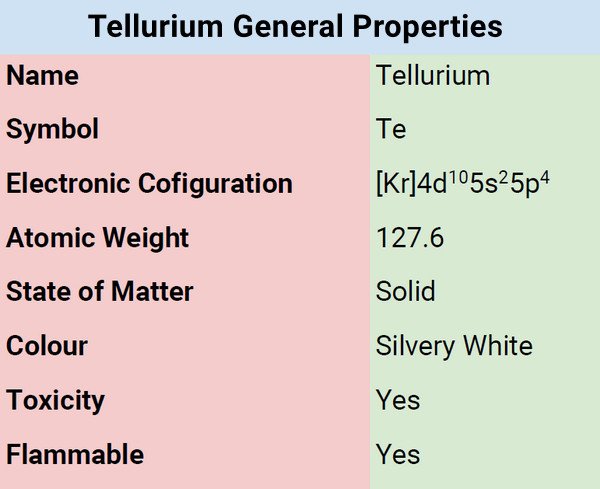
Tellurium is a metalloid characterized by silvery-white color with a metallic sheen in its pure form. Tellurium is brittle and corrodes iron, copper, and stainless steel when it is molten. Tellurium has thirty recognized isotopes. When in pure form, it contains eight isotopes. It acts as a semiconductor of electricity. It is harmful to handle the element and its compounds and so caution must be observed at all times.
Lewis Dot Structure of Tellurium

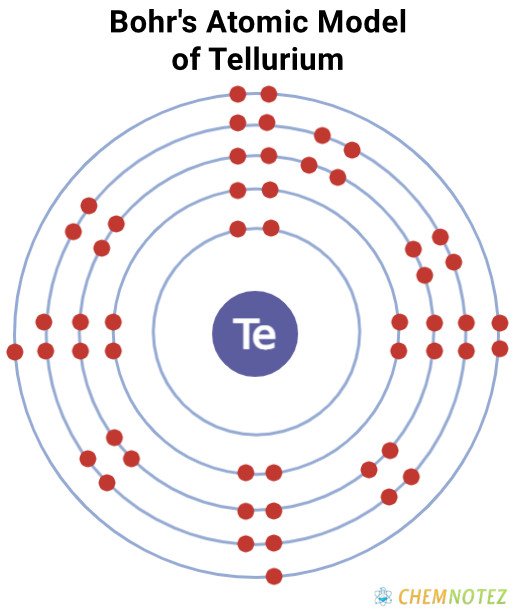
Bohr’s Atomic model of Tellurium
Atomic Data of Tellurium
Physical Properties of Tellurium
| Color | Gray/Silvery-white |
| Odor | Garlic-like odor |
| Taste | Tasteless |
| Atomic Mass | 127.60 |
| Weight | 127.60 |
| Density | 6.232 |
| Atomic Radius | 2.06 Å |
| Ionization Energy | 869.294kJ mol−1 |
| Covalent Radius | 1.37Å |
| Ionic Radius | 0.221 nm (-2) ; 0.089 nm (+4) |
| Electronic Gain Enthalpy | 190.161 kJ mol−1 |
| Electron Negativity | 2.1 |
| Electron Affinity | 190.161 kJ mol−1 |
| Melting Point | 449.51°C, 841.12°F, 722.66 K |
| Boiling Point | 988°C, 1810°F, 1261 K |
Chemical Properties of Tellurium
| Atomic Number | 52 |
| Group | 16 |
| Period | 5 |
| Block | p |
| Electronic Configuration | [Kr] 4d105s25p4 |
| Combustion | Flammable |
| Chemical Reactivity | Unreactive |
| Valency of Element | 6 |
Different States of Tellurium
As a metalloid, tellurium is solid at room temperature. It does melt once the desired temperature is reached. When it comes to oxidation states, it appears in various oxidation states with +4 as the most common.
Uses of Tellurium
- Tellurium is added to alloys to increase their machinability and strength, usually in combination with copper, lead, and stainless steel.
- It is used to vulcanize rubber.
- It is used to tint glass and ceramics.
- It acts as a catalyst in oil refining.
- It is useful in semiconductor applications for it can be doped with gold, copper, silver, or tin. As a semiconductor, it shows exceptional electrical conductivity in various directions or when exposed to light.
- Tellurium is useful in making blasting caps.
- Bismuth telluride is used in various thermoelectric devices.
- Far-infrared detectors use lead telluride.
- Tellurium is essential in the manufacturing of optical modulators, memory chips, and solar panels.
- Tellurium is used in making rubber because of its ability to make rubber resistant to heat.
- Tellurium is one of the components used in manufacturing computers and calculators.
- Tellurium is used in petroleum cracking.
- The tellurium compound in the form of tellurium suboxide is useful in the manufacturing of DVDs and CDs.
- The tellurium compound in the form of tellurite agar is useful in the identification of pathogens, specifically the one that causes diphtheria.
- Tellurium is used in cast iron alloy for chill testing and control.
Price of Tellurium
The cost of tellurium fluctuates depending on the supply and demand. Pure tellurium costs around $24 per 100 grams. The price is significantly lower if you purchase in bulk.
Interesting facts about Tellurium
- The origin of the word Tellurium is tellus, which is the Latin word for Earth.
- Don’t you know that tellurium is the least common on earth? The average tellurium in most rock formations is about 3 parts per billion. This makes tellurium a rare earth element.
- Tellurium is eight times less abundant than gold.
- The leading producer of tellurium in the world is China.
- Tellurium is one of the lightest elements to portray alpha decay.
- One particular compound of tellurium, molten tellurium can corrode stainless steel, copper, and iron.
- The representation of tellurium is an earth-like sphere, indicating where the name tellurium originated.
Frequently Asked Questions
Q1. Is tellurium dangerous to humans?
Tellurium is not dangerous to humans, but if they come into touch with it, they may develop health problems like fatigue, a metallic taste in their tongue, dryness of the mouth, and a distinct garlic-like odor. Additionally, nausea, vomiting, and constipation could occur.
Q2. Does the human body use tellurium?
There is no known biological function for tellurium. When it is metabolized by the body, it produces dimethyl telluride, which is a volatile gaseous compound. The body will get rid of it through exhalation and sweating. It is the cause of a charmingly potent garlic breath.
Q3. What foods contain tellurium?
Tellurium can be consumed along with meals like nuts, seafood, and some dairy items. It is present in many fatty foods, and some plants. Tellurium is not present in considerable quantities in either the ambient air or drinking water.
Q4. Can tellurium be recycled?
Yes. Some anode slimes used in the copper refining process are recovered for their tellurium content. Additionally, it is recycled from damaged and outdated solar panels. Production waste from the production of BiTe is another recycling stream.
Q5. Is tellurium water soluble?
Tellurium is not soluble in water. It does, however, soluble in some acids and solutions like potassium cyanide solution, potassium hydroxide, nitric acid, and sulfuric acid.
References
- https://www.ccdc.cam.ac.uk/Community/educationalresources/PeriodicTable/Tellurium/
- https://www.rsc.org/periodic-table/element/52/tellurium
- https://www.lenntech.com/periodic/elements/te.htm
- https://www.britannica.com/science/tellurium
- https://www.livescience.com/37396-tellurium.html
- https://byjus.com/chemistry/tellurium/
- https://sciencestruck.com/tellurium-facts
- https://study.com/academy/lesson/what-is-tellurium-facts-properties-discovery.html
- https://www.thoughtco.com/tellurium-facts-606602
- https://www.chemicool.com/elements/tellurium.html