What is Zinc?
Zinc is one of the chemical elements essential to life. Of the elements in the metal group, zinc is one of the most widely used. It is a transition metal used every day. In the human body, a trace of zinc plays an integral part in the enzyme carbonic anhydrate, especially at high concentrations in red blood cells. Let’s know more about this element!
Where is Zinc obtained?
Zinc is not available in pure form. It is found in the minerals in the earth’s crust, which makes zinc the 24th in the list of most abundant elements on earth. Ocean water and air have traces of zinc. Minerals mined for zinc are hemimorphite, smithsonite, sphalerite, and wurtzite. Of all these, sphalerite is commonly mined because it contains a high level of zinc. Australia, Peru, and China are the major producer of zinc.
History of Zinc
Zinc is one of the few elements known to Romans, but they rarely use it. Zinc was the first metal in India. In the period of 1100 to 1500, zinc was refined until China made the move to refine zinc on a large scale. In 1745, a ship from the East India Company sank and it carried a cargo of Chinese zinc. An analysis showed that it was pure metal. P. Moras de Respour, a Flemish metallurgist, extracted zinc from zinc oxide in 1668. However, there is a different version of it in Europe. For them, Andreas Marggraf, a German chemist, was the one who discovered zinc in 1746.

Classification, Properties, and Characteristics of Zinc
At room temperature, zinc is classified as hard metal characterized by its bluish-white color. It is also known for its hardness. When it reaches a particular temperature (100 degrees Celsius), it gets more malleable and less brittle. When it comes to boiling and melting points, zinc has a relatively low boiling and melting point. When it comes to electrical conductivity, it is somewhat fair. When gets in contact with air, it develops a reaction with carbon dioxide. It forms a thin layer of zinc carbonate, which protects zinc from reacting further.
Lewis Dot Structure of Zinc

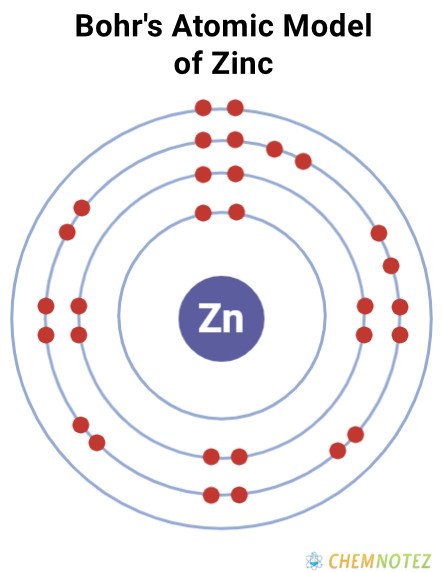
Bohr’s Atomic Model of Zinc
Atomic Data of Zinc
Physical Properties of Zinc
| Color | Silvery White Metal with Blue Tinge/Bluish-white |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 65.38 |
| Weight | 65.39 |
| Density | 7.133 grams/cm3 at 25 °C (68 °F) |
| Atomic Radius | 2.01 Å |
| Ionization Energy | 906.402 kJ mol−1 |
| Covalent Radius | 1.20 Å |
| Ionic Radius | 0.074 nm (+2) |
| Electronic Gain Enthalpy | -58(20) kJ/mol |
| Electron Negativity | 1.65 |
| Electron Affinity | -0.6(2) eV |
| Melting Point | 420 °C (788 °F) |
| Boiling Point | 907 °C (1,665 °F) |
Chemical Properties of Zinc
| Atomic Number | 30 |
| Group | 12 |
| Period | 4 |
| Block | d |
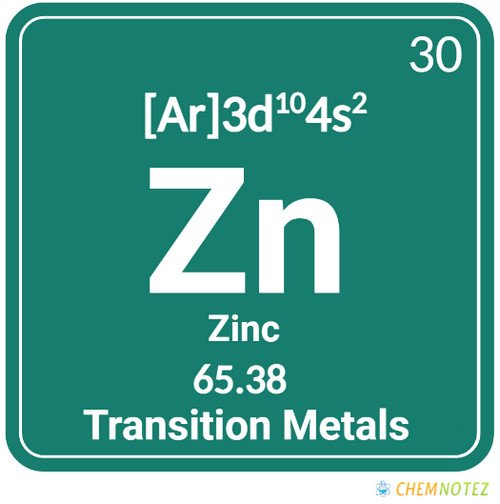
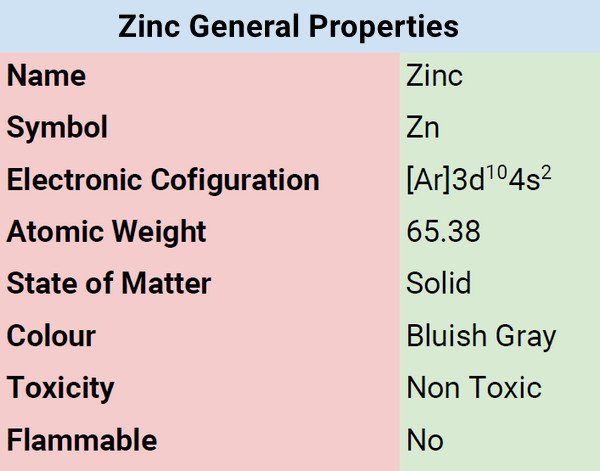
| Electronic Configuration | [Ar]3d104s2 |
| Combustion | Highly Flammable |
| Chemical Reactivity | Highly Reactive |
| Valency of Element | 2 |
Different States of Zinc
Zinc, as a metal, is solid at room temperature. If you put ore zinc to heat, it releases free metal. However, zinc sublimates easily, in other words, it changes from solid to gas once heated. It’s one of the unique states of zinc – the ability to turn from solid to gas – bypassing the liquidation state.

Uses of Zinc
- Zinc is used to galvanize other metals for the purpose of rust prevention. The end product is used to make street lamp posts, bodies of cars, suspension bridges, and safety barriers.
- Zinc in large quantities is used to produce die-castings, which is essential in various industries such as electrical, automobile, and hardware.
- Zinc plays a vital role in making alloys like nickel, brass, aluminum, and silver.
- Zinc compounds in the form of zinc oxide are used in the production of various products like plastics, paints, pharmaceuticals, cosmetics, rubber, textiles, soaps, batteries, and some types of electrical equipment.
- Zinc sulfide is used to make x-ray screens, fluorescent lights, and luminous paints.
- Galvanization of iron is done with the help of zinc to prevent corrosion.
- Zinc is a vital trace element for plants and animals. It helps maintain the balance of enzymes in humans and improves the body’s immune system.
Price of Zinc
The cost of zinc is dependent on supply and demand. Pure zinc costs $5.30 per 100 grams, but the price is cheaper if you purchase it in bulk.
Interesting facts about Zinc
- Don’t you know that zinc has a healing mechanism? Its coating serves as a protection of base steel.
- Don’t you know that the earth’s crust has around 0.004% zinc?
- Zinc is the 24th most abundant element on the earth.
- Almost all life forms contain zinc. An average healthy adult has around 2 grams of zinc in the body.
- Children need zinc to grow. In adults, zinc is needed to maintain good health and boost reproduction.
- Zinc can improve eyesight.
- Zinc is the second most common trace element found in the body.
- Don’t you know that zinc acts as a natural insect repellent? It also protects the skin and lips.
- Don’t you know that zinc is 100% recyclable? In fact, 1/3 of zinc consumed in North America is from recyclable materials.
- Don’t you know that zinc can make the usual vehicle last longer?
- Don’t you know that zinc has the ability to store six times more energy when compared to other battery systems? It significantly increases the range of electric-powered cars. A vehicle powered by zinc-air batteries has a speed of 120 mph.
- Don’t you know that brass is formed when combining zinc and copper?
- The United States penny is made up of 98% zinc and copper coating.
- Don’t you know that if you burn zinc, it emits a bright bluish-to-greenish flame?
- Some food sources are rich in zinc such as nuts, mustards, wheat, and edible seeds like sesame seeds.
- Some toothpaste and baby powder have zinc.
- Prestal, a metal alloy consists of 22% aluminum and 78% zinc. It acts as plastic but is ultra-strong. In fact, its toughness is the same as steel.
Frequently Asked Questions about Zinc
Q1. Why does zinc have a low melting point?
Zinc has fully filled the d-orbital. Its size is greater when compared with Iron, and its valence electrons are less confined to the nucleus. Hence, the reasons why zinc has a low melting point.
Q2. Does zinc rust in water?
when zinc is exposed to air and water, it corrodes, which is a typical characteristic of ferrous metal. However, its corrosion on steel has a rate of 1/30. Zinc rusts at varying rates. it all depends on the environment to which zinc is exposed.
Q3. What is zinc best known for?
Zinc is known for its significance in carrying almost 100 enzymes, which are vital for various chemical reactions in the body. It plays a primary role in DNA creation, cellular growth, building proteins, boosting the immune system, and improving the healing process.
Q4. What happens to the body if you consumed too much zinc?
Anything that is too much is bad. If you consume too much zinc, it causes undesirable symptoms like loss of appetite, nausea and vomiting, diarrhea, headaches, and abdominal cramps.
Q5. What is the greatest source of zinc?
Oysters contain a high level of zinc. Although you can find zinc in meat and poultry products.
References
- https://galvanizeit.org/hot-dip-galvanizing/what-is-zinc/facts-about-zinc
- https://www.britannica.com/science/zinc
- https://www.rsc.org/periodic-table/element/30/zinc
- https://www.lenntech.com/periodic/elements/zn.htm
- https://www.thoughtco.com/interesting-zinc-element-facts-603359
- https://www.chemicool.com/elements/zinc.html
- https://www.livescience.com/29378-zinc.html
- https://byjus.com/chemistry/zinc/
- https://www.medicalnewstoday.com/articles/263176
- https://ods.od.nih.gov/factsheets/Zinc-Consumer/