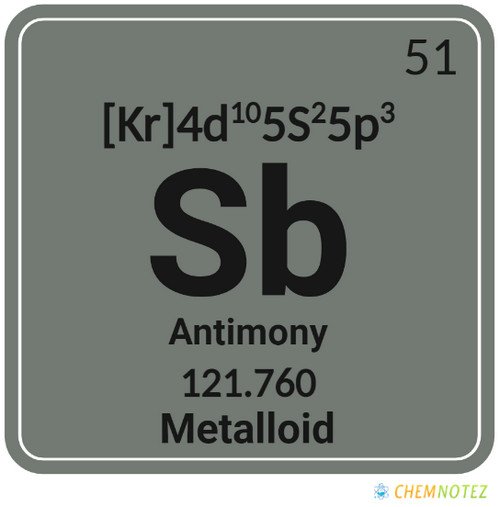
What is Antimony?
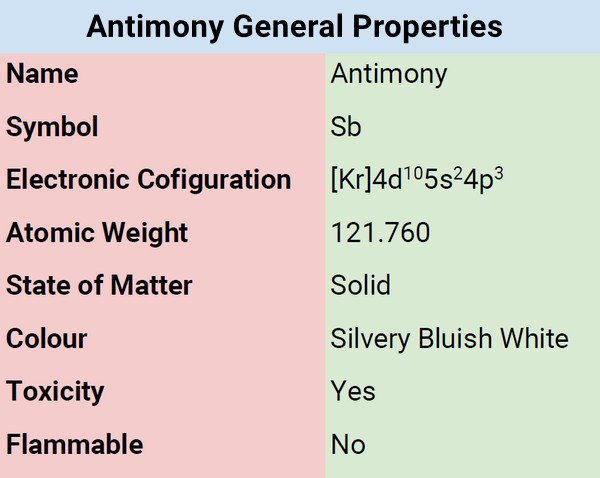
Antimony is part of the nitrogen group (Group 15). It is a metallic element that comes in a variety of allotropic forms. Antimony is a glossy, silvery, bluish-white solid with a flaky texture. It is extremely fragile. Mostly, it appears as the gray sulfide mineral stibnite.
Where is Antimony obtained?
Although antimony is a rare element, it can be found in trace amounts in more than 100 different types of minerals. Most frequently, it is discovered as antimony(III) sulfide. It is obtained through the process of roasting the antimony(III) sulfide to the oxide state and subsequently reducing it with carbon,. More so, about 88% of the antimony in the world is made in China, Tajikistan, Russia, and Bolivia.
History of Antimony
The ancients were aware of antimony and its compounds, and there is a 5,000-year-old antimony vase in the Louvre in Paris. During the 16th century BC, an Egyptian papyrus made mention of antimony sulfide (Sb2S3). This pigment’s naturally occurring black form, known as khol, was previously applied as mascara. The temptress Jezebel was known for using this type of mascara.

Yellow lead antimonite was another color used by the Chaldean (inhabitants of Southern Iraq) in the sixth and seventh century BC. This was discovered in the glazing of decorative bricks in Babylon and dates from Nebuchadnezzar’s reign. In Medieval Times, antimony was widely used to harden lead. Some people took it as a laxative pill.
Classification, Properties and Characteristics of Antimony
Antimony belongs to the metalloid group of chemical elements, and because of its metalloid status, antimony exhibits certain metallic characteristics but is not sufficient to be categorized as a genuine metal. It physically behaves like sulfur but the chemical structure is similar to that of the typical metal element.
The conductivities of antimony are less than those of most metals in both electrical and thermal directions. The most usual characteristic of antimony is crystalline solid, brittle, and flammable. In addition, antimony has the peculiar quality of expanding as it freezes, just like water.
Lewis Dot Structure of Antimony
Atomic Data of Antimony
Physical Properties of Antimony
| Color | Silvery bluish/white |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 121.760 |
| Weight | 121.76 u |
| Density | 6.68 |
| Atomic Radius | 2.06 Å |
| Ionization Energy | 830.583kJ mol−1 |
| Covalent Radius | 1.40Å |
| Ionic Radius | 0.245 nm (-3); 0.062 nm (+5); 0.076 nm (+3) |
| Electronic Gain Enthalpy | 100.924 kJ mol−1 |
| Electron Negativity | 2.05 |
| Electron Affinity | 100.924 kJ mol−1 |
| Melting Point | 630.628°C, 1167.13°F, 903.778 K |
| Boiling Point | 1587°C, 2889°F, 1860 K |
Chemical Properties of Antimony
| Atomic Number | 51 |
| Group | 15 |
| Period | 5 |
| Block | p |
| Electronic Configuration | [Kr] 4d105s25p3 |
| Combustion | Can be flammable in powder and dust form |
| Chemical Reactivity | Fairly Reactive |
| Valency of Element | 5 |
Different States of Antimony
Antimony is a metalloid element that is solid at room temperature. It melts once desired temperature is reached.

Uses of Antimony
- Antimony is widely used in the electronics sector, specifically in the manufacturing of semiconductor devices.
- It is used to alloy other metals like lead to significantly increase its strength and toughness.
- Alloys consisting of lead and antimony are used in batteries, cable sheathing, and bullets.
- Some antimony compounds are used to make pants, pottery, glass, enamels, and other flame-retardant materials.
- Antimony alloys are useful in manufacturing typefaces for printing. The result is clear and sharp printing.
- Materials used for machinery bearings like Babbit metals are made from metal alloys like copper, tin, lead, and antimony primarily because of their strength and slippery characteristics.
Price of Antimony
The cost of antimony fluctuates depending on supply and demand. Pure antimony costs around $4.5 per 100 grams. It costs cheaper if you purchase in bulk.
Interesting facts about Antimony
- Don’t you know that the name antimony was derived after anti and monos, Greek words meaning “a metal not found alone?” It is named that way because antimony always exists with another chemical element.
- Antimony’s chemical symbol “Sb” was derived from its historical name “stibium.” It comes from the Greek word “stibi”, which means “mark.” Looking back at antimony’s history, it was previously used to make black eye makeup.
- Don’t you know that antimony has biblical references? In the Old Testament, Queen Jezebel used a compound of antimony for makeup purposes.
- You should not inhale or ingest antimony because it can be poisonous. Although antimony has been used for various medicinal purposes such as laxatives. It is not practiced today because of the potential health danger associated with antimony.
- Don’t you know that one of the presumed reasons for Wolfgang Amadeus Mozart’s early death was antimony? It is believed that his doctor poisoned him using a toxic antimony drug.
Frequently Asked Questions
Q1. Is antimony dangerous to humans?
Antimony may possess a danger to living creatures. Humans can develop antimony poisoning secondary to prolonged exposure to antimony. The adverse consequences of antimony exposure can include gastrointestinal problems, antimony skin spots, respiratory irritation, and pneumoconiosis. Additionally, antimony trioxide has been linked to human cancer.
Q2. How does antimony get into the body?
There are various ways for antimony to get through the body such as through the food you eat and water you drink. It can also enter the body via inhalation of air or dust particles that may be contaminated with antimony.
Q3. What can antimony bond with?
Antimony is reactive to almost all metals on the periodic table. It reacts with other metals to form pnictides, which have five valence electrons. Three of these electrons are in the p shell and unpaired while the other two electrons are paired and in the subshell.
Q4. What makes antimony toxic?
The toxicity of antimony is linked with the enzyme combination that seems to interfere with various cellular metabolism.
Q5. Does antimony burn in the air?
Halogens and sulfur when heated can oxidize antimony. When you heat antimony in air, it burns a bright blue flame and gives off trioxide Sb203, which is acid and alkali-soluble.
References
- https://www.rsc.org/periodic-table/element/51/antimony
- https://www.britannica.com/science/antimony
- https://www.lenntech.com/periodic/elements/sb.htm
- https://www.livescience.com/37390-antimony.html
- https://www.chemicool.com/elements/antimony.html
- https://www.thoughtco.com/antimony-element-facts-606498
- https://en.wikipedia.org/wiki/Antimony
- https://study.com/learn/lesson/antimony-symbols-uses-facts.html
- https://byjus.com/chemistry/antimony/
- https://www.psanalytical.com/information/antimony.html