What is Palladium?
Palladium is a priceless gray-white metal that is incredibly ductile and simple to handle. It belongs to the platinum metal group together with osmium, ruthenium, iridium, and platinum. At normal temperatures, palladium does not tarnish.
Its structure is face-centered cubic crystalline and exhibits strong resistance to acid attack and air corrosion. Strong acids attack it, and aqua regia dissolves it. Numerous chemicals and complicated salts are produced by it.
Where is Palladium obtained?
In nature, palladium has been discovered uncombined in Brazil, however, the majority of it is present in sulfide minerals like braggite. It is a nickel refining byproduct that is commercially extracted. It is additionally extracted as a by-product of the processing of copper and zinc.
History of Palladium
Brazilian miners in 1700 were aware of a natural alloy of palladium and gold. They named it ouro podre, or “worthless gold. However, palladium was originally isolated from platinum, a feat accomplished in 1803 by William Wollaston, rather than from this source. He observed that not all of the common platinum dissolved in aqua regia.
He eventually retrieved palladium from the residue it left behind. Instead of making his finding public, he offered the new metal for sale under the name “new silver.” Richard Chenevix bought some, looked into it, and proclaimed it to be a platinum and mercury alloy. Wollaston disclosed his identity as the metal’s discoverer in February 1805 and provided a thorough and compelling description of the metal and its characteristics.

Classification, Properties and Characteristics of Palladium
Palladium is characterized by its glossy, silvery-white color. It belongs to the platinum metal group. It is low in toxicity. Just like gold, it is ductile, malleable, and can be beaten into thin leaf. When exposed to air, it remains untarnished but will tarnish gradually when exposed to moist air that contains sulfur.
It is resistant to corrosion except when exposed to nitric acid at the usual temperature. When in compounds, palladium usually exists in the II oxidation state. It is remarkable in its ability to absorb up to 900 times its own hydrogen volume. It expands as it absorbs hydrogen, which is comparable to a sponge when it absorbs water.
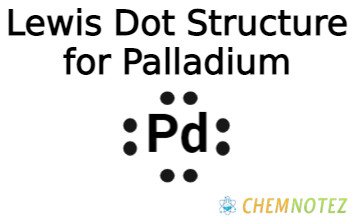
Lewis Dot Structure of Palladium
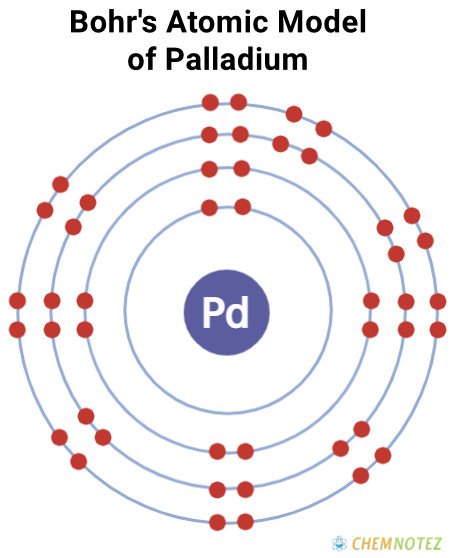
Bohr’s Atomic Model of Palladium
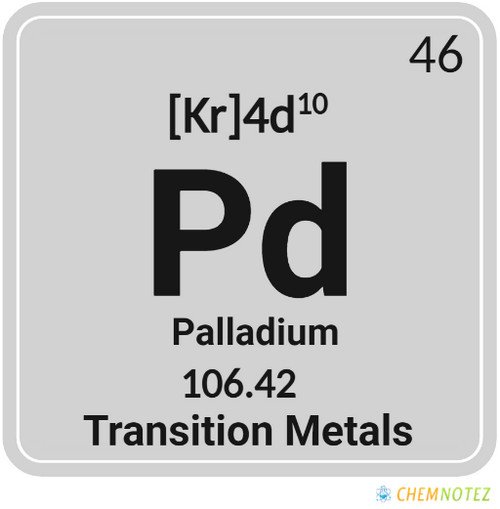
Atomic Data of Palladium
Physical Properties of Palladium
| Color | Gray White/Silvery White |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 106.42 |
| Weight | 106.40 |
| Density | 12.0 |
| Atomic Radius | 2.10Å |
| Ionization Energy | 804.389kJ mol−1 |
| Covalent Radius | 1.30Å |
| Ionic Radius | 0.137 nm |
| Electronic Gain Enthalpy | 54.225kJ mol−1 |
| Electron Negativity | 2.20 |
| Electron Affinity | 54.225kJ mol−1 |
| Melting Point | 1,554.9 °C (2,830.8 °F) |
| Boiling Point | 2,963 °C (5,365 °F) |
Chemical Properties of Palladium
| Atomic Number | 46 |
| Group | 10 |
| Period | 5 |
| Block | d |
| Electronic Configuration | [Kr]4d10 |
| Combustion | Non-flammable |
| Chemical Reactivity | Reactive |
| Valency of Element | -2 |
Different States of Palladium
Palladium is a transition metal, which remains solid at room temperature. It usually exists in 0 and +2 states, although some oxidation states can also be found.

Uses of Palladium
- It is commonly used as a catalytic converter for vehicles because of its efficiency in removing partially burnt and unburnt hydrocarbons from fuel.
- Palladium is used for making jewelry.
- It is used to make dental crowns and dental fillings.
- An alloy of gold, white gold, is decolorized by alloying another metal, which sometimes includes palladium.
- Palladium is used in the electronics sector, specifically in ceramic capacitors commonly found in mobile phones and laptop computers. It has palladium layers sandwiched between ceramic layers.
- Palladium is used in hydrogenation and dehydrogenation reactions. Through heated palladium, hydrogen will easily diffuse, which will pave the way to separate and purify the gas.
- Palladium is resistant to corrosion, which makes it a very good alloy in low-voltage electrical contact.
- Palladium is used in springs, watch bearings, and balance wheels.
- The mirrors used in scientific instruments are made from palladium.
- Many electrical appliances today use palladium such as wide-screen televisions and computers.
- Palladium is one of the materials used in electroplating.
- Some surgical instruments are made using palladium.
Price of Palladium
Palladium is one of the most expensive precious metals. It costs around $5,800 per 100 grams, although the price is lower if you purchase in bulk.
Interesting facts Palladium
- Don’t you know that the name palladium was derived from the asteroid Pallas, the Greek Goddess of wisdom? She is also the granddaughter of Poseidon.
- Palladium is one of the precious metals that have an ISO currency code. Its codes are XPD and 964. Other metals that have ISO currency codes are platinum, silver, and gold.
- Palladium has the capacity to absorb hydrogen. In fact, its hydrogen absorption ability is 900 times more than other metals.
- Although palladium is biologically inactive, it can still trigger allergic reactions in some people. Although for the most part, palladium is hypoallergic. Therefore, it is generally safe to use by everyone.
- Palladium is one of the non-toxic metals.
- Don’t you know that 18ct white gold has palladium? It is alloyed to gold to give it a beautiful white color. That is why the price of white gold is not dependent on the market value of the gold alone, but also on the fluctuating price of palladium.
Frequently Asked Questions
Q1. What substances can palladium react with?
Palladium is highly reactive to oxygen and hydrogen. Its surfaces are a good catalyst for chemical reactions, especially in the hydrogenation of unsaturated organic compounds.
Q2. How poisonous is palladium?
When compared to other metals, palladium is extremely low in toxicity. In fact, the body poorly absorbs it when ingested. In a very rare instances, it may cause eye irritation, skin irritation, and respiratory tract issues.
Q3. Will a magnet stick to palladium?
Palladium does not have a magnetic property. The magnetic test is one of the methods used to detect if the palladium is pure or not. There are palladium materials, especially in jewelry that are not really 100% palladium. They are made using iron and palladium to save money because iron is an inexpensive metal, not to mention, it is easy to mold too. you will know if it is not pure palladium if it will react to a magnet. Why? It is because iron reacts to magnets.
Q4. Does palladium last forever?
Palladium is naturally bright white. It does not fade so there is no need to re-plate it. Yes, its color lasts forever. That is why it belongs to the group of precious metals.
Q5. What are the disadvantages of palladium?
If there is one disadvantage of palladium, it would be its price. It is expensive. In fact, more expensive than platinum. One good thing is that because of its low density, less is needed to create a particular piece of jewelry. Hence, you will be able to save money if you have a piece of jewelry made from palladium because only a few of it is needed to craft a beautiful jewelry piece.
References
- https://www.britannica.com/science/palladium-chemical-element
- https://www.rsc.org/periodic-table/element/46/palladium
- https://www.lenntech.com/periodic/elements/pd.htm
- https://www.chemicool.com/elements/palladium.html
- https://www.thoughtco.com/palladium-facts-606573
- https://www.livescience.com/36991-palladium.html
- https://study.com/learn/lesson/palladium-element-facts.html
- http://www.chemistryexplained.com/elements/L-P/Palladium.html
- https://lambdageeks.com/palladium-chemical-properties/
- https://byjus.com/chemistry/palladium/