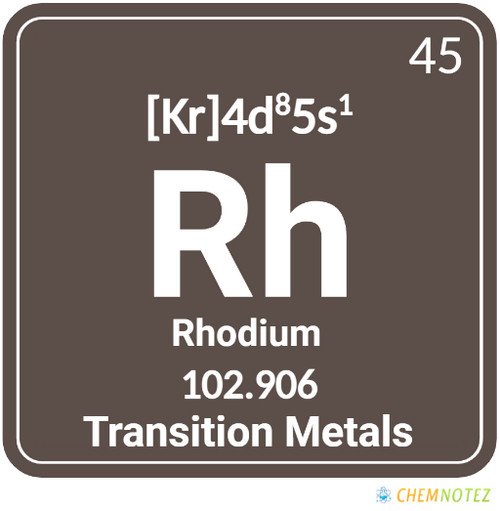
What is Rhodium?
Silver-white rhodium is a metallic element with exceptional reflectivity and corrosion resistance. It belongs to the platinum group metals. It ranks well beyond gold or silver as the rarest and most expensive precious metal on the entire planet. Rhodium, which is named for the rose-red hue of its salts, derives its name from the “rhodon”, a Greek word for rose. Compared to platinum, rhodium has a lower density and a higher melting point. When heated, it turns red and changes back to an element at higher temperatures.
Where is Rhodium obtained?
Rhodium is the least common non-radioactive metal. It naturally occurs in river sands in North and South America, just like other platinum metals. Rhodium is also present in the Canadian province of Ontario’s copper-nickel sulfide ores. Rhodium is produced in commercial quantities as a by-product of the refining of copper and nickel. About 30 tonnes are produced globally each year.
History of Rhodium
William Wollaston discovered rhodium in 1803. He worked with Smithson Tennant on a business project that included making pure platinum for sale. Ordinary platinum was first dissolved in aqua regia (a solution of nitric and hydrochloric acids). It didn’t dissolve completely, and a dark residue was left behind. He focused on dissolved platinum and palladium solutions.

By precipitating these metals out, he was left with a stunning red solution, which he used to make crystals that were rose-red in color, which is now known as sodium rhodium chloride. from them, he was able to create a sample of rhodium.
Classification, Properties and Characteristics of Rhodium
The metal rhodium is characterized by its silvery-white color. The metal gradually transforms into sesquioxide if exposed to red heat in the atmosphere. It reverts to its elemental state at higher temperatures. Compared to platinum, rhodium has a lower density and a higher melting point. Rhodium has a specific gravity of 12.41 (20°C), a melting point of 1966 +/-3°C, and a boiling point of 3727 +/-100°C.
Lewis Dot Structure of Rhodium

Bohr’s Atomic Model of Rhodium

Atomic Data of Rhodium
Physical Properties of Rhodium
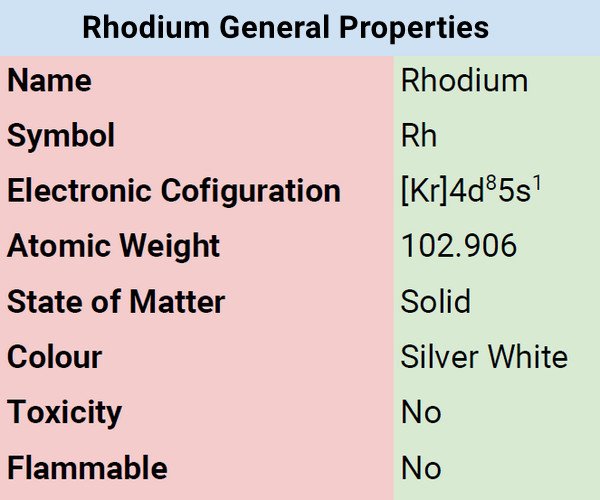
| Color | Silvery White |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 102.906 |
| Weight | 102.9055 u |
| Density | 12.4 |
| Atomic Radius | 2.10 Å |
| Ionization Energy | 719.675kJ mol−1 |
| Covalent Radius | 1.34Å |
| Ionic Radius | 68 (+3e) |
| Electronic Gain Enthalpy | 109.704 kJ mol−1 |
| Electron Negativity | 2.28 |
| Electron Affinity | 109.704 kJ mol−1 |
| Melting Point | 1963°C, 3565°F, 2236 K |
| Boiling Point | 3695°C, 6683°F, 3968 K |
Chemical Properties of Rhodium
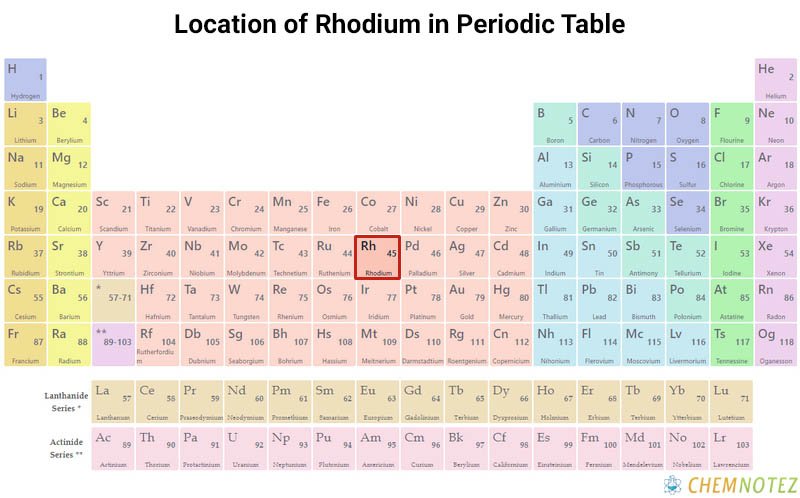
| Atomic Number | 45 |
| Group | 9 |
| Period | 5 |
| Block | d |
| Electronic Configuration | [Kr] 4d85s1 |
| Combustion | Highly Flammable |
| Chemical Reactivity | Inert to reaction |
| Valency of Element | 21 |
Different States of Rhodium
Rhodium, as a transition metal, is solid at room temperature. Although it can be purchased in liquid in diluted or concentrated form. It has several oxidation states with +3 as the most common.

Uses of Rhodium
- Rhodium is mostly used (80%) in automotive catalytic converters to significantly decrease nitrogen oxides in exhaust emissions. It also serves as a catalyst in various industrial processes.
- In the chemical industry, rhodium serves as a catalyst in various chemical processes such as nitric acid, acetic acid, and hydrogenation processes.
- Rhodium is used to cover crucibles, headlight reflectors, optical mirrors, and thermocouple elements, to name a few.
- Due to its low electrical resistance and good corrosion resistance, it is utilized as an electrical contact material.
- It acts as an alloying agent to greatly harden metals like palladium and platinum.
- Plated rhodium is known for its high reflectance and durability, thus, the reason why it is used to make jewelry pieces, optical instruments, and other decorative pieces.
Price of Rhodium
Of all precious metals, rhodium is the most expensive. It cost around $13,000 per 100 grams. You’ll be able to save money if you purchase in bulk. Although the price fluctuates depending on supply and demand. But even so, it is still expensive, but definitely worth the price.
Interesting facts about Rhodium
- Don’t you know that one of the materials used for heart pacemakers is an alloy of rhodium and platinum?
- Rhodium has the ability to resist most acids.
- Rhodium is rarely used in pure form. It is always used as an alloy.
- Rhodium, out of all metals used in vehicle catalytic converters, is the most effective in removing nitrogen oxides from the exhaust.
- Rhodium compounds easily decompose or reduce through the process of heating.
- Rhodium is derived from the Greek word “rhodon”, which means “rose.”
- Of all non-radioactive metals, rhodium is the rarest.
- The top producers of rhodium are Zimbabwe, Russia, and South Africa.
- Rhodium does not have any biological significance.
- Rhodium only has one electron in the outer shell, contrary to other elements that belong to group 9.
- Rhodium only forms one part per 200 million in the crust of the earth.
- Rhodium is one of the toughest precious metals to melt because of its high melting point and lower density. This only goes to show that the supply of rhodium is short making it all the more expensive.
- Pure rhodium is non-toxic, but then becomes part of a compound, it can be highly toxic. Therefore, you should handle rhodium cautiously.
- Don’t you know that rhodium is used to coat white gold making it appear shiny and appear similar to that silver, but with an obviously higher value than silver?
Frequently Asked Questions
Q1. Is rhodium harmful to humans?
Breathing in rhodium powder can have negative effects on the body. it can also cause irritation to the eyes and skin. once rhodium powder gets in contact with the skin, there is a possibility of skin allergies such as rashes and itching.
Q2. What makes rhodium so valuable?
There are many reasons why rhodium is valuable and one of which is its rarity. Rhodium is worth twice as much as gold when compared to the market price of gold. It is a rare precious metal that is more valuable than gold and other precious metals.
Q3. What is rhodium weakness?
The drawbacks of utilizing rhodium as a heavy catalyst of metal are its high cost, sensitivity to competing for C-H insertions, and lack of stereocontrol.
Q4. How much rhodium is left in the world?
There are about 644,000 ounces of rhodium left in the world as of May 2022 making rhodium one of the most scarce and most experienced precious metals.
Q5. What can replace rhodium?
In comparison to platinum, palladium is less expensive, lighter, and has oxidation resistance on par with rhodium. Due diligence has consequently been put into determining its worth as a significant addition to high-temperature platinum alloys.
References
- https://www.rsc.org/periodic-table/element/45/rhodium
- https://www.britannica.com/science/rhodium
- https://www.livescience.com/36988-rhodium.html
- https://www.thoughtco.com/rhodium-facts-606586
- https://www.lenntech.com/periodic/elements/rh.htm
- https://www.chemicool.com/elements/rhodium.html
- https://study.com/academy/lesson/what-is-rhodium-facts-history-isotopes.html
- https://pubchem.ncbi.nlm.nih.gov/compound/Rhodium
- https://chemistrytalk.org/rhodium-element/
- https://byjus.com/chemistry/rhodium/