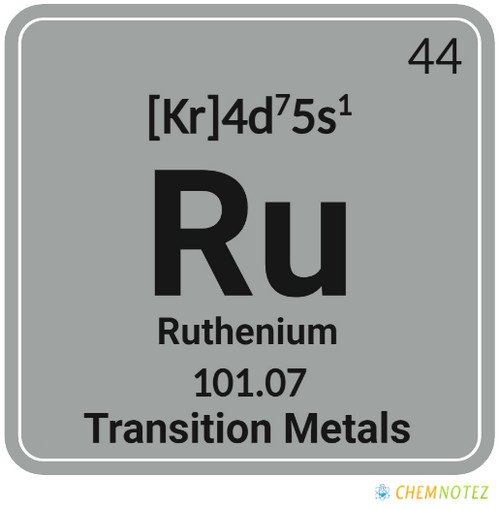
What is Ruthenium?
Ruthenium is a bright, silvery-white metal, usually found in small amounts as a minor component of platinum ore. It is extremely inert. If exposed to high temperatures, ruthenium will oxidize. Ruthenium is a very rare element that makes up roughly 100 parts per trillion of the Earth’s crust, ranking it as the 74th most abundant element. Only 12 tons of ruthenium are mined each year, and it is extracted from the scrap after refining nickel.
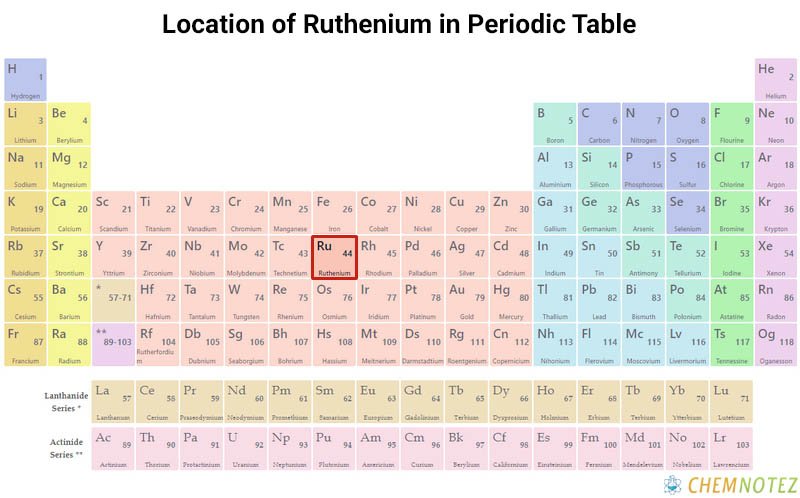
Where is Ruthenium obtained?
The Ural Mountains, as well as North and South America, contain ruthenium along with other platinum group metals. Additionally, it can be found in South African pyroxenite deposits in the nickel mining district of Sudbury, Ontario. Radioactive waste can also be used to extract ruthenium.
Isolating ruthenium involves a difficult process. Ruthenium is produced as a by-product during the electrorefining and reduction of nickel, copper, or platinum ores. Ruthenium is kept during the process in anode mud. The mud is combined with sodium peroxide, and the resulting combination is then treated to aqua-regia.
History of Ruthenium
In May 1808, Jedrzej Sniadecki, a Polish chemist, was researching platinum ores from South America when he made the discovery of a new metal, which he named vestium. French chemists attempted to replicate his discovery, but they were not able to locate it in their platinum ore. Sniadecki withdrew his claim after realizing he had been misinformed about it.

Platinum from the Ural Mountains was examined by Gottfried Osann of the University of Dorpat, Baltic in 1825. He was able to discover three new elements and reported them as polinium, pluranium, and ruthenium. out of the three, only ruthenium was verified. It was later isolated, refined, and certified by Karl Karlovich Klaus at the University of Kazan in 1840.
Classification, Properties and Characteristics of Ruthenium
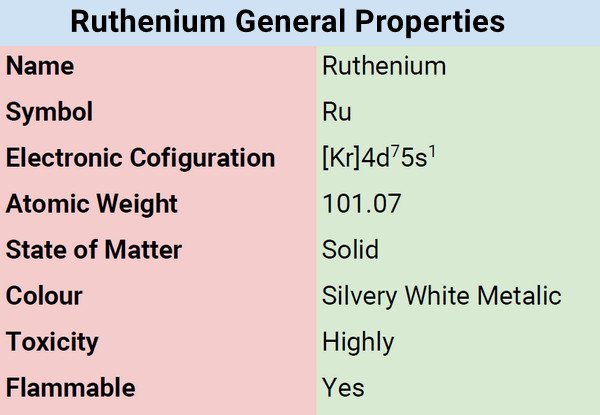
Ruthenium is an extremely rare, fragile, hard, glossy, silvery-white metal that does not corrode when left out in the open.
Ruthenium, like other transition metals, can occur in a variety of oxidation states, with oxidation states II, III, and IV being the most prevalent.
Acids, water, and air have no effect on the metal. It oxidizes explosively when in contact with halogens and molten alkali.
Lewis Dot Structure of Ruthenium

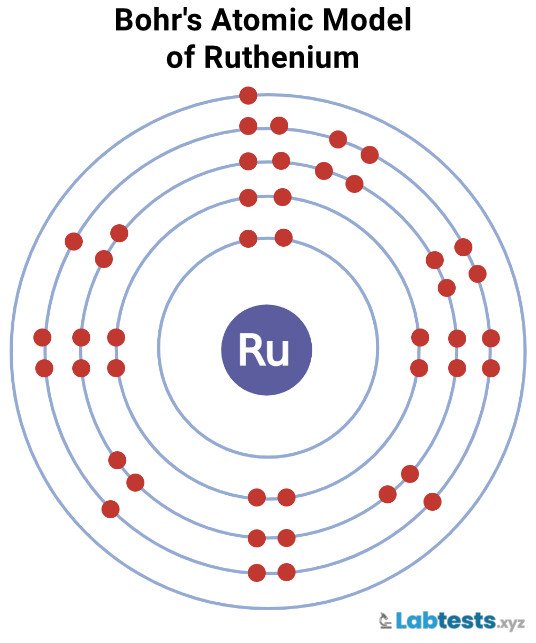
Bohr’s Atomic Model of Ruthenium
Atomic Data of Ruthenium
Physical Properties of Ruthenium
| Color | Silvery White |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 101.07 |
| Weight | 101.07 |
| Density | 12.1 grams per cubic centimeter |
| Atomic Radius | 2.13Å |
| Ionization Energy | 710.18kJ mol−1 |
| Covalent Radius | 1.36Å |
| Ionic Radius | 67 (+4e) |
| Electronic Gain Enthalpy | 101.31kJ mol−1 |
| Electron Negativity | 2.2 |
| Electron Affinity | 101.31kJ mol−1 |
| Melting Point | 2607 K (2334°C or 4233°F) |
| Boiling Point | 4423 K (4150°C or 7502°F) |
Chemical Properties of Ruthenium
| Atomic Number | 44 |
| Group | 8 |
| Period | 5 |
| Block | d |
| Electronic Configuration | [Kr] 4d75s1 |
| Combustion | Flammable |
| Chemical Reactivity | Relatively Unreactive |
| Valency of Element | 4d7 5s1 |
Different States of Ruthenium
Ruthenium, which is categorized as a transition metal, is solid at ambient temperature. It liquefies once desired temperature is reached, which is 2334°C.

Uses of Ruthenium
- The majority of ruthenium is utilized in the electronics sector for electrical connections and chip resistors.
- The anodes of electrochemical cells used to produce chlorine in the chemical industry are coated with ruthenium oxide.
- It is used as a catalyst for the synthesis of acetic acid and ammonia using ruthenium.
- Solar cells, which convert light energy into electrical energy, can use ruthenium molecules.
- It acts as platinum and palladium hardeners. Ruthenium is alloyed with these metals to provide electrical connections with extremely high wear resistance.
- In certain jewelry, it is combined with platinum as an alloy.
- Other metals are plated with ruthenium. The most typical metals utilized to create ruthenium coatings are those formed through electrodeposition or thermal decomposition.
- Some pen nibs contain ruthenium. A perfect example is a parker pen nib like Parker 51. Its nib is marked “RU”, which means it contains 96% ruthenium and the rest is iridium. (Avoid biting on your pen!)
- Titanium’s resistance is 100 times better when added with 0.1% ruthenium.
Price of Ruthenium
The cost of ruthenium is dependent on supply and demand. The cost of pure ruthenium is around $1400 per 100 grams. If you purchase in bulk, you will be able to save money. Bulk purchase of ruthenium costs around $650 per 100 grams.
Interesting facts about Ruthenium
- Of all metals in the platinum group, ruthenium was the last to be discovered.
- Ruthenium comes from the Latin word “Ruthenia”, which means Russia for the reason that the original source of platinum metal group ore is the Ural Mountains of Russia.
- Ruthenium can combine to generate compounds that resemble those of cadmium.
- Ruthenium is hazardous to humans, just like cadmium. It is thought to be carcinogenic. RuO4 (ruthenium tetroxide) is regarded as highly hazardous.
- Compounds of ruthenium can discolor or stain the skin.
- Don’t you know that out of all the chemical elements in group 8, ruthenium is the only one that doesn’t have two electrons in the outer shell?
- Halogens and hydroxides can attack ruthenium in a pure state. Acids, water, and air have no effect on it.
- Although ruthenium can exist in a variety of oxidation states (7 or 8), the II, III, and IV states are the most frequent.
- Ruthenia is the Latin name for Russia where the word ruthenium originates.
Frequently Asked Questions
Q1. Is Ruthenium toxic?
Ruthenium, its radioisotope Ru-106, as well as its derivatives, have the potential to be carcinogenic, posing long-term health risks. They can also react violently with other chemicals. The metal should be handled carefully because ingesting it could induce progressive accumulation of the metal in the bones and its vapors could irritate the eyes and mouth as well as the skin and lungs.
Q2. Can ruthenium burn forever?
Ruthenium has been identified as the cause of the unending fires in Southern Turkey. For more than 2500 years, the flames also referred to as Yanartas, have been burning. Since ruthenium can nearly burn indefinitely when coupled with methane, scientists now think that ruthenium is partially to blame for this.
Q3. Is ruthenium a good conductor of heat?
Heat and electricity do not conduct well through ruthenium. It has a high melting point and the ability to resist corrosion.
Q4. Can you recycle ruthenium?
Yes. In fact, the recycling rate of ruthenium is high and it’s primarily a result of ruthenium being recovered and used in catalytic applications.
Q5. How do you remove ruthenium from gold?
one way of separating ruthenium from gold is by dipping the object (gold) in Aqua Regia, which is a mixture of nitric acid and hydrochloric acid. what this mixture does is it dissolves gold leaving only ruthenium and other insoluble components.
References
- https://www.industrialheating.com/blogs/14-industrial-heating-experts-speak-blog/post/94522-facts-about-the-elements-ruthenium
- https://www.rsc.org/periodic-table/element/44/ruthenium
- https://www.thoughtco.com/ruthenium-facts-ru-element-606589
- https://www.britannica.com/science/ruthenium
- https://www.chemicool.com/elements/ruthenium.html
- https://www.livescience.com/34836-ruthenium.html
- https://www.lenntech.com/periodic/elements/ru.htm
- https://periodic-table.com/ruthenium/
- https://byjus.com/chemistry/ruthenium/
- https://www.chemistrylearner.com/ruthenium.html