What is Tin?
Tin belongs to Group 14 (IVa) of the periodic also known as the carbon family. The ancients used this metal a long time ago, but they thought of it as bronze. It typically used as copper alloy. Tin is soft, silvery-white with a bluish undertone, and ranked fourth in the order in the periodic table of elements, just under the post-transition metal group.
Where is Tin obtained?
The mineral cassiterite is the main source of tin in the crust of the Earth. It rarely appears in its natural state. It ranks at about 50th in terms of abundance in the crust of the Earth. The “tin belt,” which consists of countries that runs across Asian countries like China, Indonesia, and Thailand is where it is primarily found. Additionally, it is mined in Brazil, Peru, and Bolivia. Tin is commercially produced by reducing the ore using the coal in the furnace.
History of Tin
It is believed that the Chinese were mining tin in Yunnan Province around 700 BC. Pure tin was also found at Machu Picchu. Tin’s beginnings can be found in the prehistoric Bronze Age, which lasted from 3300 BCE to 1200 BCE. Tin was utilized at this time to create bronze, an alloy of copper (80%) and tin (12%).

Classification, Properties and Characteristics of Tin
Tin is suitable for all types of cold-working, including rolling, spinning, and extrusion. It is malleable, nontoxic, and ductile. Tin in pure form has the ability to retain its color after exposure because a thin, invisible stannic oxide film spontaneously forms by interaction with the oxygen in the air, which serves as the tin’s protective layer.
Tin’s low melting point and strong adherence to clean steel, iron, copper, and copper alloy surfaces make it an ideal material for use as an oxidation-resistant coating. Tin is resistant to water-induced corrosion. This makes it possible to utilize it as a plating substance to shield other metals.
Lewis Dot Structure of Tin

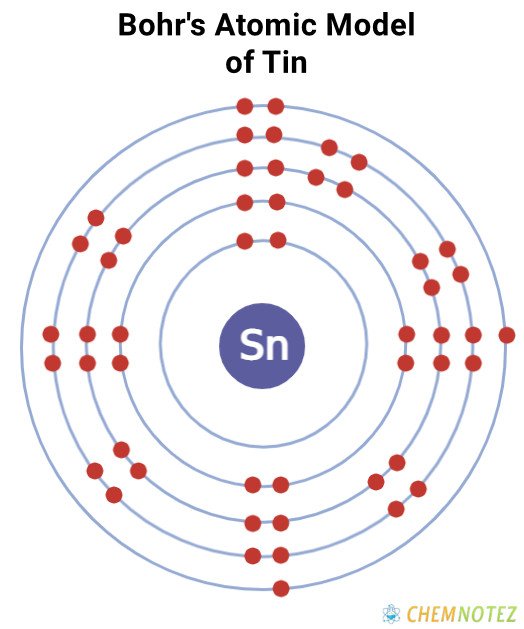
Bohr’s Atomic Model of Tin
Atomic Data of Tin
Physical Properties of Tin
| Color | Silvery Gray/Silvery White |
| Odor | Odorless |
| Taste | Tasteless |
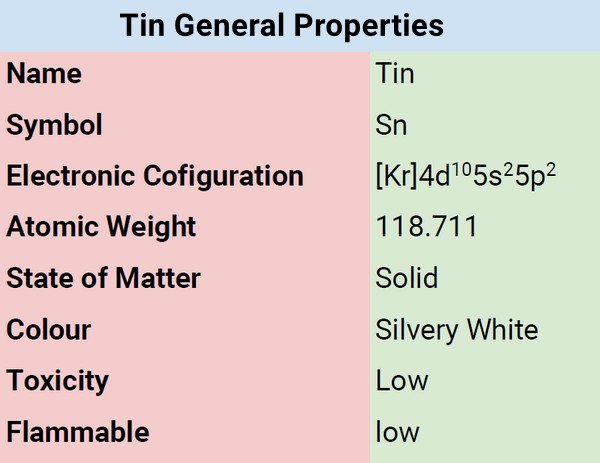
| Atomic Mass | 118.710 |
| Weight | 118.69 |
| Density | 7.287 |
| Atomic Radius | 2.17 Å |
| Ionization Energy | 708.581 kJ mol−1 |
| Covalent Radius | 1.40 Å |
| Ionic Radius | 0.112 nm (+2) ; 0.070 nm (+4) |
| Electronic Gain Enthalpy | 107.298 kJ mol−1 |
| Electron Negativity | 1.96 |
| Electron Affinity | 107.298 kJ mol−1 |
| Melting Point | 231.97 °C (449.54 °F) |
| Boiling Point | 2,270 °C (4,100 °F) |
Chemical Properties of Tin
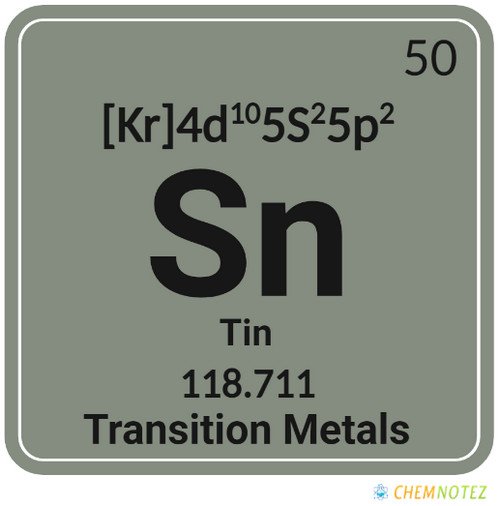
| Atomic Number | 50 |
| Group | 14 |
| Period | 5 |
| Block | p |
| Electronic Configuration | [Kr]4d105s25p2 |
| Combustion | Non-flammable but may ignite in powder form |
| Chemical Reactivity | Relatively Reactive |
| Valency of Element | 4 |
Different States of Tin
Tin belongs to the metal group, which is typically solid at room temperature. It is subjected to melting once desired temperature is reached, which is around 449.47 degrees Fahrenheit. It exists in two oxidation states, which are +2 and +4 with elemental tin usually oxidizes at 2+ in an acidic solution.

Uses of Tin
- Niobium-tin alloy is one of the materials used to create superconducting magnets.
- Window glass have floating molten glass over molten tin to achieve a uniform flat finish. Glass is treated with tin salts to create coatings that are electrically conductive.
- A particular tin compound; tin oxide is one of the components used for making gas sensors and ceramics.
- Tin chloride, a tin salt acts as a reducing agent and a mordant for dyeing silk and calico.
- Another tin compound, zinc stannate is used as a fire retardant in some plastic materials.
- Some tin compounds are used as an anti-fouling paint for boats and ships, although some countries already banned their use because of the potential danger to aquatic life.
- One of the common uses of tin today is to create solder, which is a mixture of tin and lead and is primarily used to make electronic circuits and join pipes.
- Tin is a very good plating material. It acts as a protective coating against other metals like steel and zinc.
- Tin acts as a metal alloy for pewter and bronze.
- It plays an important role in manufacturing textiles. Tin chloride acts as a mordant in dyeing textiles. It is also used to significantly increase silk’s weight.
- Tin-plated steel containers are used to extend the food’s shelf life.
- Stannous fluoride, which is a tin compound is used in the production of some types of toothpaste.
- It acts as a reducing and coloring agent for pottery and sensors.
- It is used in battery electrodes such as Li-ion batteries.
Price of Tin
The cost of tin fluctuates depending on supply and demand. Pure tin costs around $24 per 100 grams. The cost is cheaper if you purchase in bulk.
Interesting facts about Tin
- Tin got its name from the Anglo-Saxon language.
- Tin’s symbol, Sn is derived from stannun, which is the Latin word for tin.
- Don’t you know that if you bend a tin bar, it makes a screaming sound fondly referred to as “tin cry?” The sound is produced as the atom’s crystal structure breaks.
- Don’t you know that if the temperature falls below 13.2 degrees Celsius, the color of white tin changes to gray? To prevent the changing of color, a small impurity is added to the white tin.
- Don’t you know that bronze is 12% tin?
- Don’t you know that foil was once made from tin material? Yes, the same wrapping material is used to wrap foods and drugs. Although it is not replaced by aluminum foil.
Frequently Asked Questions
Q1. Is tin harmful to humans?
Inorganic tin compounds get inside and outside the body through inhalation and eating, and when this happens, it rarely causes harm. However, if you consumed or inhaled a large number of inorganic tin compounds, you can experience some sort of discomfort such as stomach ache, anemia, renal issues, and liver-related problems.
Q2. What happens when the tin gets cold?
When the temperature gets severely low exposing tin to an extremely cold environment, pure tin will eventually decompose and turns to dust.
Q3. Is tin a safe metal?
Tin is non-toxic, which makes it a safe metal. In fact, if you happen to consume a small amount of tin, it rarely causes gastrointestinal issues.
Q4. Is tin used to prevent rusting?
There are various ways to prevent rusting and corrosion and one of which is tinning. It is the process of applying a thin layer of tin on the metal’s surface, which is common in fabricated iron and steel.
Q5. How long does tin lining last?
Tin has a long lifespan. With proper care and maintenance, it can last for 10 years or even longer. cookware containing tin material lasts for many years to come with occasional upkeep.
References
- https://www.britannica.com/science/tin
- https://www.rsc.org/periodic-table/element/50/tin
- https://www.ducksters.com/science/chemistry/tin.php
- https://www.lenntech.com/periodic/elements/sn.htm
- https://www.thoughtco.com/tin-facts-606608
- https://www.chemicool.com/elements/tin.html
- https://www.livescience.com/37355-tin.html
- https://en.wikipedia.org/wiki/Tin
- https://www.vedantu.com/evs/facts-about-tin
- https://pubchem.ncbi.nlm.nih.gov/element/Tin