What is Xenon?
One of the noble gases, xenon, is chemically non-reactive. Although not hazardous by itself, its components are potent oxidizers that are extremely toxic. More interesting facts about xenon are discussed below.
Where is Xenon obtained?
Xenon is found in the air, with 0.086 parts per million in terms of volume. It can be observed in the vapors that arise from specific mineral springs. It is extracted from liquid air for commercial use.

History of Xenon
William Ramsay and Morris Travers made the discovery of xenon in July 1898 at University College London. They were unsure if liquid air included any other gases because they had already extracted krypton, and argon from it. They employed a fresh liquid-air machine given to them by Ludwig Mond, a wealthy entrepreneur. They use it to extract more krypton.

They ultimately separated a heavier gas by continuous distillation, and when they looked at this in a vacuum tube, it gave out a lovely blue glow. They understood it to be yet another member of the gaseous elements known at the time as the “inert” group because of their low chemical reactivity.
The new gas was given the name xenon. Neil Bartlett eventually demonstrated that this gas was not inert by creating a fluorine derivative in 1962. Over 100 different xenon compounds have so far been created.
Classification, Properties and Characteristics of Xenon
Xenon is a heavy, uncommon gas that has no color, taste, or smell. It belongs to the inert gas family. For the most part, xenon is inert to chemicals. Today, a wide variety of xenon compounds have been created, primarily using fluorine or oxygen Two xenon oxides that are extremely explosives are xenon tetroxide and xenon trioxide.
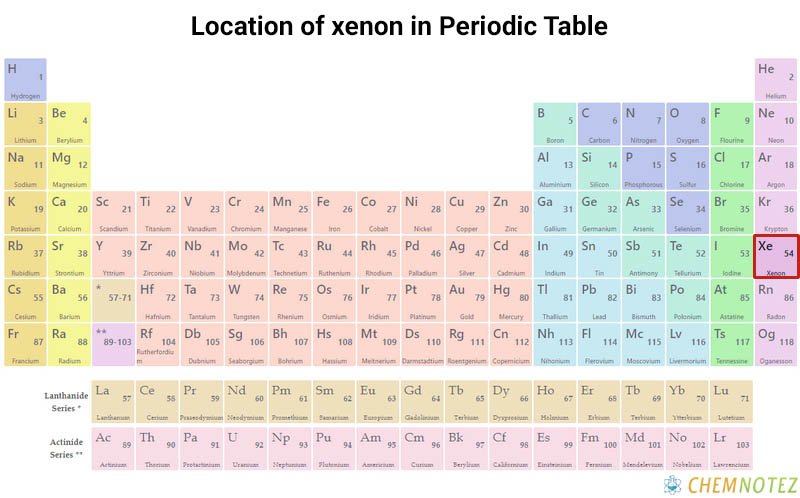
Lewis Dot Structure of Xenon
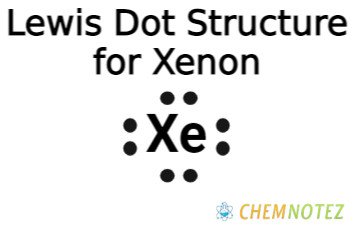
Bohr’s Atomic Model
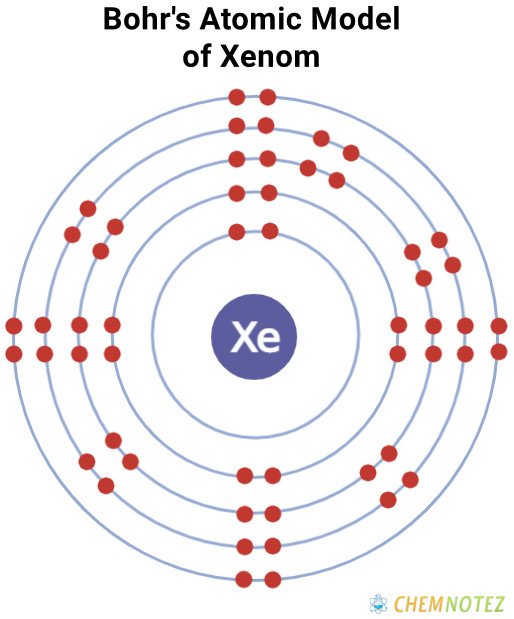
Atomic Data of Xenon
Physical Properties of Xenon
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Atomic Mass | 131.293 |
| Weight | 131.29 g.mol -1 |
| Density | 0.005366 |
| Atomic Radius | 2.16 (Å |
| Ionization Energy | 1170.352 kJ mol−1 |
| Covalent Radius | 1.36 Å |
| Ionic Radius | 216 pm |
| Electronic Gain Enthalpy | Unknown |
| Electron Negativity | 2.60 |
| Electron Affinity | Unknown |
| Melting Point | −111.75°C, −169.15°F, 161.4 K |
| Boiling Point | −108.099°C, −162.578°F, |
Chemical Properties of Xenon
| Atomic Number | 54 |
| Group | 18 |
| Period | 5 |
| Block | p |
| Electronic Configuration | [Kr] 4d105s25p6 |
| Combustion | Non-flammable |
| Chemical Reactivity | Non-reactive |
| Valency of Element | Zero |
Different States of Xenon
Xenon is a noble or so-called inert gas. In fact, it was the very first noble gas that forms real chemical compounds.

Uses of Xenon
- One of the uses of xenon is in specialized light sources. It radiantly glows a blue color when excites by an electrical discharge.
- Xenon lamps are popular because they have high-speed electronic flash bulbs commonly used by photographers.
- Lamps used in food processing and preparation use xenon such as bactericidal lamps and sunbed lamps.
- Ruby lasers use xenon lamps.
- Some satellites use xenon ion propulsion systems to keep them in orbit. Some spacecraft use the same system too.
- Xenon difluoride is primarily used to etch microprocessors made from a silicon material.
- Xenon is one of the components used in manufacturing 5-fluorouracil, which is a drug used in the management of cancer.
- Photographers used xenon in photographic flashes such as in high-pressure arc lamps, which is widely used in motion picture projection. Xenon is also used to produce ultraviolet light in high-pressure arc lamps.
- Xenon is used in instruments to detect radiation such as x-ray counters, neutron, and bubble chambers.
- Xenon has medicinal importance. It is used in medical imaging and in general anesthetic. It is specifically used to image vital organs of the body such as the lungs, brain, and heart.
- Xenon and other inert gases are used by modern ion thrusters for space travel. It is used for the propellant to eliminate the risk of explosion related to chemical propulsion.
- A mixture of xenon and oxygen is used as inhalants to create a hormone that helps increase the production of red blood cells.
- Xenon is used in MR Spectroscopy.
- Xenon in high-pressure lamps creates a light that is the same as the sun. hence, they can be used to mimic solar light for lab testing of solar panels.
- Xenon is used to project pictures and images in films for movie projectors.
Price of Xenon
The cost of xenon is dependent on supply and demand. Pure xenon costs around $120 per 100 grams. The price is lower if you purchase in bulk.
Interesting facts about Xenon
- Don’t you know that you can create a deep-sounding voice with the help of xenon? It is because xenon is denser than air. The sound it produces is the opposite of the sound produced by helium.
- Don’t you know that if you use xenon to fill a balloon, the balloon will not float? It will only sink to the floor.
- The origin of the name xenon is Xenos, a Greek word for a stranger.
- Of all elements that belong to the gas family, xenon is the most expensive.
- If you electrify xenon, it produces a bluish-purple color.
- Xenon when combined with fluorine forms excellent compounds.
- Xenon is added to compressed air tanks of deep-sea divers to prevent nitrogen accumulation in the blood. When nitrogen accumulates in the blood, it forms fatal gas bubbles when divers return to land.
Frequently Asked Questions
Q1. What is xenon attracted to?
Xenon is reactive to highly electronegative and minute-sized oxygen and fluorine, which is essential in the formation of xenon compounds.
Q2. What makes xenon glow?
Xenon creates a blue or light lavender glow when electrically charged because the excited electrons leave its electron shell and travel around the atom’s nucleus at a higher state.
Q3. How long do xenon lights Last?
Lights powered by xenon are called high-intensity discharge lamps. They are three times brighter than halogen lights. They are energy efficient and can last for up to 2,500 hours.
Q4. Can humans breathe xenon?
Xenon is tagged as a simple asphyxiate. If you happen to inhale a high concentration of xenon, you can experience a variety of symptoms such as nausea, vomiting, dizziness, loss of consciousness, and even death.
Q5. Do xenon lights get hot?
Xenon lights don’t get too hot, which makes them a perfect accent light, especially for lighting beneath cabinets.
References
- https://www.livescience.com/37504-facts-about-xenon.html
- https://www.rsc.org/periodic-table/element/54/xenon
- https://www.britannica.com/science/xenon
- https://www.thoughtco.com/facst-about-the-noble-gas-xenon-609608
- https://www.chemicool.com/elements/xenon.html
- https://www.lenntech.com/periodic/elements/xe.htm
- https://sciencenotes.org/xenon-facts-and-uses-atomic-number-54-element-symbol-xe/
- https://www.factsjustforkids.com/chemistry-facts/xenon-facts-for-kids/
- https://en.wikipedia.org/wiki/Xenon
- https://study.com/academy/lesson/what-is-xenon-definition-uses-facts.html